Chronic oxidative DNA damage due to DNA repair defects causes chromosomal instability in Saccharomyces cerevisiae
- PMID: 18591251
- PMCID: PMC2519736
- DOI: 10.1128/MCB.00307-08
Chronic oxidative DNA damage due to DNA repair defects causes chromosomal instability in Saccharomyces cerevisiae
Erratum in
-
Correction for Degtyareva et al., "Chronic Oxidative DNA Damage Due to DNA Repair Defects Causes Chromosomal Instability in Saccharomyces cerevisiae".Mol Cell Biol. 2019 Oct 11;39(21):e00407-19. doi: 10.1128/MCB.00407-19. Print 2019 Nov 1. Mol Cell Biol. 2019. PMID: 31604842 Free PMC article. No abstract available.
Abstract
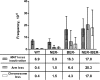
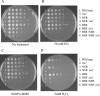
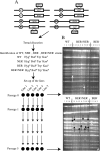
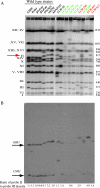
Oxidative DNA damage is likely to be involved in the etiology of cancer and is thought to accelerate tumorigenesis via increased mutation rates. However, the majority of malignant cells acquire a specific type of genomic instability characterized by large-scale genomic rearrangements, referred to as chromosomal instability (CIN). The molecular mechanisms underlying CIN are not entirely understood. We utilized Saccharomyces cerevisiae as a model system to delineate the relationship between genotoxic stress and CIN. It was found that elevated levels of chronic, unrepaired oxidative DNA damage caused chromosomal aberrations at remarkably high frequencies under both selective and nonselective growth conditions. In this system, exceeding the cellular capacity to appropriately manage oxidative DNA damage resulted in a "gain-of-CIN" phenotype and led to profound karyotypic instability. These results illustrate a novel mechanism for genome destabilization that is likely to be relevant to human carcinogenesis.
Figures


Similar articles
-
Genome-wide map of Apn1 binding sites under oxidative stress in Saccharomyces cerevisiae.Yeast. 2017 Nov;34(11):447-458. doi: 10.1002/yea.3247. Epub 2017 Sep 26. Yeast. 2017. PMID: 28752642 Free PMC article.
-
Reduced levels of DNA polymerase delta induce chromosome fragile site instability in yeast.Mol Cell Biol. 2008 Sep;28(17):5359-68. doi: 10.1128/MCB.02084-07. Epub 2008 Jun 30. Mol Cell Biol. 2008. PMID: 18591249 Free PMC article.
-
Spontaneous DNA damage in Saccharomyces cerevisiae elicits phenotypic properties similar to cancer cells.J Biol Chem. 2004 May 21;279(21):22585-94. doi: 10.1074/jbc.M400468200. Epub 2004 Mar 12. J Biol Chem. 2004. PMID: 15020594
-
Wrestling off RAD51: a novel role for RecQ helicases.Bioessays. 2008 Apr;30(4):291-5. doi: 10.1002/bies.20735. Bioessays. 2008. PMID: 18348153 Review.
-
Role of genomic instability in arsenic-induced carcinogenicity. A review.Environ Int. 2013 Mar;53:29-40. doi: 10.1016/j.envint.2012.12.004. Epub 2013 Jan 8. Environ Int. 2013. PMID: 23314041 Review.
Cited by
-
Yap1: a DNA damage responder in Saccharomyces cerevisiae.Mech Ageing Dev. 2012 Apr;133(4):147-56. doi: 10.1016/j.mad.2012.03.009. Epub 2012 Mar 17. Mech Ageing Dev. 2012. PMID: 22433435 Free PMC article.
-
Post-replication repair suppresses duplication-mediated genome instability.PLoS Genet. 2010 May 6;6(5):e1000933. doi: 10.1371/journal.pgen.1000933. PLoS Genet. 2010. PMID: 20463880 Free PMC article.
-
Adaptation of organisms by resonance of RNA transcription with the cellular redox cycle.PLoS One. 2011;6(9):e25270. doi: 10.1371/journal.pone.0025270. Epub 2011 Sep 28. PLoS One. 2011. PMID: 21980411 Free PMC article.
-
Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii.PLoS One. 2014 Nov 12;9(11):e112660. doi: 10.1371/journal.pone.0112660. eCollection 2014. PLoS One. 2014. PMID: 25391025 Free PMC article.
-
Piecing Together How Peroxiredoxins Maintain Genomic Stability.Antioxidants (Basel). 2018 Nov 28;7(12):177. doi: 10.3390/antiox7120177. Antioxidants (Basel). 2018. PMID: 30486489 Free PMC article. Review.
References
-
- Al-Tassan, N., N. H. Chmiel, J. Maynard, N. Fleming, A. L. Livingston, G. T. Williams, A. K. Hodges, D. R. Davies, S. S. David, J. R. Sampson, and J. P. Cheadle. 2002. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat. Genet. 30227-232. - PubMed
-
- Augeri, L., Y. M. Lee, A. B. Barton, and P. W. Doetsch. 1997. Purification, characterization, gene cloning, and expression of Saccharomyces cerevisiae redoxyendonuclease, a homolog of Escherichia coli endonuclease III. Biochemistry 36721-729. - PubMed
-
- Balakumaran, B. S., C. H. Freudenreich, and V. A. Zakian. 2000. CGG/CCG repeats exhibit orientation-dependent instability and orientation-independent fragility in Saccharomyces cerevisiae. Hum. Mol. Genet. 993-100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
