GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium
- PMID: 18591257
- PMCID: PMC2519727
- DOI: 10.1128/MCB.00717-08
GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium
Abstract
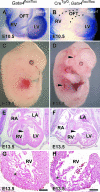
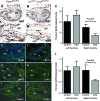
The anterior heart field (AHF) comprises a population of mesodermal progenitor cells that are added to the nascent linear heart to give rise to the majority of the right ventricle, interventricular septum, and outflow tract in mammals and birds. The zinc finger transcription factor GATA4 functions as an integral member of the cardiac transcription factor network in the derivatives of the AHF. In addition to its role in cardiac differentiation, GATA4 is also required for cardiomyocyte replication, although the transcriptional targets of GATA4 required for proliferation have not been previously identified. In the present study, we disrupted Gata4 function exclusively in the AHF and its derivatives. Gata4 AHF knockout mice die by embryonic day 13.5 and exhibit hypoplasia of the right ventricular myocardium and interventricular septum and display profound ventricular septal defects. Loss of Gata4 function in the AHF results in decreased myocyte proliferation in the right ventricle, and we identified numerous cell cycle genes that are dependent on Gata4 by microarray analysis. We show that GATA4 is required for cyclin D2, cyclin A2, and Cdk4 expression in the right ventricle and that the Cyclin D2 and Cdk4 promoters are bound and activated by GATA4 via multiple consensus GATA binding sites in each gene's proximal promoter. These findings establish Cyclin D2 and Cdk4 as direct transcriptional targets of GATA4 and support a model in which GATA4 controls cardiomyocyte proliferation by coordinately regulating numerous cell cycle genes.
Figures




References
-
- Abu-Issa, R., and M. L. Kirby. 2007. Heart field: from mesoderm to heart tube. Annu. Rev. Cell Dev. Biol. 2345-68. - PubMed
-
- Adams, P. D. 2001. Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim. Biophys. Acta 1471M123-M133. - PubMed
-
- Adams, P. D., and W. G. Kaelin, Jr. 1995. Transcriptional control by E2F. Semin. Cancer Biol. 699-108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
