The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts
- PMID: 18591259
- PMCID: PMC2519714
- DOI: 10.1128/MCB.00563-08
The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts
Abstract
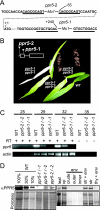
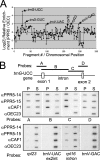
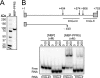
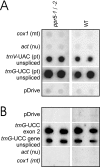
Genes for pentatricopeptide repeat (PPR) proteins are found in all eukaryotic genomes analyzed but are particularly abundant in land plants. The majority of analyzed PPR proteins play a role in the processing or translation of organellar RNAs. Few PPR proteins have been studied in detail, and the functional repertoire and mechanisms of action of proteins in the PPR family are poorly understood. Here we analyzed a maize ortholog of the embryo-essential Arabidopsis thaliana gene AtPPR5. A genome-wide analysis of chloroplast RNAs that coimmunoprecipitate with Zea mays PPR5 (ZmPPR5) demonstrated that ZmPPR5 is bound in vivo to the unspliced precursor of trnG-UCC. Null and hypomorphic Zmppr5 insertion mutants are embryo viable but are deficient for chloroplast ribosomes and die as seedlings. These mutants show a dramatic decrease in both spliced and unspliced trnG-UCC RNAs, while the transcription of trnG-UCC is unaffected. These results, together with biochemical data documenting the sequence-specific binding of recombinant PPR5 to the trnG-UCC group II intron, suggest that PPR5 stabilizes the trnG-UCC precursor by directly binding and protecting an endonuclease-sensitive site. These findings add to the evidence that chloroplast-localized PPR proteins that are embryo essential in Arabidopsis typically function in the biogenesis of the plastid translation machinery.
Figures




References
-
- Barkan, A. 1998. Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 29738-57.
-
- Barkan, A., and M. Goldschmidt-Clermont. 2000. Participation of nuclear genes in chloroplast gene expression. Biochimie 82559-572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
