Functional cloning and characterization of the multidrug efflux pumps NorM from Neisseria gonorrhoeae and YdhE from Escherichia coli
- PMID: 18591276
- PMCID: PMC2533508
- DOI: 10.1128/AAC.00475-08
Functional cloning and characterization of the multidrug efflux pumps NorM from Neisseria gonorrhoeae and YdhE from Escherichia coli
Abstract
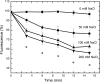
Active efflux of antimicrobial agents is one of the most important adapted strategies that bacteria use to defend against antimicrobial factors that are present in their environment. The NorM protein of Neisseria gonorrhoeae and the YdhE protein of Escherichia coli have been proposed to be multidrug efflux pumps that belong to the multidrug and toxic compound extrusion (MATE) family. In order to determine their antimicrobial export capabilities, we cloned, expressed, and purified these two efflux proteins and characterized their functions both in vivo and in vitro. E. coli strains expressing norM or ydhE showed elevated (twofold or greater) resistance to several antimicrobial agents, including fluoroquinolones, ethidium bromide, rhodamine 6G, acriflavine, crystal violet, berberine, doxorubicin, novobiocin, enoxacin, and tetraphenylphosphonium chloride. When they were expressed in E. coli, both transporters reduced the levels of ethidium bromide and norfloxacin accumulation through a mechanism requiring the proton motive force, and direct measurements of efflux confirmed that NorM behaves as an Na(+)-dependent transporter. The capacities of NorM and YdhE to recognize structurally divergent compounds were confirmed by steady-state fluorescence polarization assays, and the results revealed that these transporters bind to antimicrobials with dissociation constants in the micromolar region.
Figures
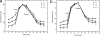
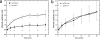
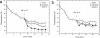


Similar articles
-
Molecular cloning and characterization of the HmrM multidrug efflux pump from Haemophilus influenzae Rd.Microbiol Immunol. 2003;47(12):937-43. doi: 10.1111/j.1348-0421.2003.tb03467.x. Microbiol Immunol. 2003. PMID: 14695443
-
NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli.Antimicrob Agents Chemother. 1998 Jul;42(7):1778-82. doi: 10.1128/AAC.42.7.1778. Antimicrob Agents Chemother. 1998. PMID: 9661020 Free PMC article.
-
An H(+)-coupled multidrug efflux pump, PmpM, a member of the MATE family of transporters, from Pseudomonas aeruginosa.J Bacteriol. 2004 Jan;186(1):262-5. doi: 10.1128/JB.186.1.262-265.2004. J Bacteriol. 2004. PMID: 14679249 Free PMC article.
-
Clamping down on drugs: the Escherichia coli multidrug efflux protein MdtM.Res Microbiol. 2018 Sep-Oct;169(7-8):461-467. doi: 10.1016/j.resmic.2017.09.006. Epub 2017 Sep 27. Res Microbiol. 2018. PMID: 28962921 Review.
-
Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis.J Mol Microbiol Biotechnol. 2001 Apr;3(2):219-24. J Mol Microbiol Biotechnol. 2001. PMID: 11321577 Review.
Cited by
-
Functionally cloned pdrM from Streptococcus pneumoniae encodes a Na(+) coupled multidrug efflux pump.PLoS One. 2013;8(3):e59525. doi: 10.1371/journal.pone.0059525. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555691 Free PMC article.
-
Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants.J Bacteriol. 2012 Nov;194(21):5941-8. doi: 10.1128/JB.00666-12. Epub 2012 Aug 31. J Bacteriol. 2012. PMID: 22942246 Free PMC article.
-
Structural insights into H+-coupled multidrug extrusion by a MATE transporter.Nat Struct Mol Biol. 2013 Nov;20(11):1310-7. doi: 10.1038/nsmb.2687. Epub 2013 Oct 20. Nat Struct Mol Biol. 2013. PMID: 24141706 Free PMC article.
-
A Novel MFS-MDR Transporter, MdrP, Employs D223 as a Key Determinant in the Na+ Translocation Coupled to Norfloxacin Efflux.Front Microbiol. 2020 May 29;11:955. doi: 10.3389/fmicb.2020.00955. eCollection 2020. Front Microbiol. 2020. PMID: 32547505 Free PMC article.
-
Legionella pneumophila strain associated with the first evidence of person-to-person transmission of Legionnaires' disease: a unique mosaic genetic backbone.Sci Rep. 2016 May 19;6:26261. doi: 10.1038/srep26261. Sci Rep. 2016. PMID: 27196677 Free PMC article.
References
-
- Begum, A., M. Rahaman, W. Ogawa, T. Mizushima, T. Kuroda, and T. Tsuchiya. 2005. Gene cloning and characterization of four MATE family multidrug efflux pumps from Vibrio cholerae non-O1. Microbiol. Immunol. 49:949-957. - PubMed
-
- Blattner, F. R., G. Plunkette III, C. A. Block, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Brooks, B. E., K. M. Piro, and R. G. Brennan. 2007. Multidrug-binding transcription factor QacR binds the bivalent aromatic diamidines DB75 and DB359 in multiple positions. J. Am. Chem. Soc. 129:8389-8395. - PubMed
-
- Brown, M. H., I. T. Paulsen, and R. A. Skurray. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31:394-395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

