Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells
- PMID: 18591406
- PMCID: PMC2442640
- DOI: 10.1084/jem.20071365
Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells
Abstract
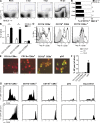
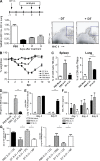
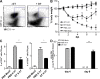
Although dendritic cells (DCs) play an important role in mediating protection against influenza virus, the precise role of lung DC subsets, such as CD11b- and CD11b+ conventional DCs or plasmacytoid DCs (pDCs), in different lung compartments is currently unknown. Early after intranasal infection, tracheal CD11b-CD11chi DCs migrated to the mediastinal lymph nodes (MLNs), acquiring co-stimulatory molecules in the process. This emigration from the lung was followed by an accumulation of CD11b+CD11chi DCs in the trachea and lung interstitium. In the MLNs, the CD11b+ DCs contained abundant viral nucleoprotein (NP), but these cells failed to present antigen to CD4 or CD8 T cells, whereas resident CD11b-CD8+ DCs presented to CD8 cells, and migratory CD11b-CD8- DCs presented to CD4 and CD8 T cells. When lung CD11chi DCs and macrophages or langerin+CD11b-CD11chi DCs were depleted using either CD11c-diphtheria toxin receptor (DTR) or langerin-DTR mice, the development of virus-specific CD8+ T cells was severely delayed, which correlated with increased clinical severity and a delayed viral clearance. 120G8+ CD11cint pDCs also accumulated in the lung and LNs carrying viral NP, but in their absence, there was no effect on viral clearance or clinical severity. Rather, in pDC-depleted mice, there was a reduction in antiviral antibody production after lung clearance of the virus. This suggests that multiple DCs are endowed with different tasks in mediating protection against influenza virus.
Figures




References
-
- Legge, K.L., and T.J. Braciale. 2005. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 23:649–659. - PubMed
-
- Pichlmair, A., O. Schulz, C.P. Tan, T.I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 314:997–1001. - PubMed
-
- Tecle, T., M.R. White, and K.L. Hartshorn. 2005. Innate immunity to influenza A virus infection. Current Respir. Med. Rev. 1:127–145.
-
- Kumagai, Y., O. Takeuchi, H. Kato, H. Kumar, K. Matsui, E. Morii, K. Aozasa, T. Kawai, and S. Akira. 2007. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 27:240–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

