Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole
- PMID: 18591669
- PMCID: PMC2453745
- DOI: 10.1073/pnas.0712241105
Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole
Abstract
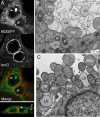
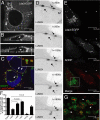
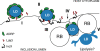
The acquisition of host-derived lipids is essential for the pathogenesis of the obligate intracellular bacteria Chlamydia trachomatis. Current models of chlamydial lipid acquisition center on the fusion of Golgi-derived exocytic vesicles and endosomal multivesicular bodies with the bacteria-containing parasitophorous vacuole ("inclusion"). In this study, we describe a mechanism of lipid acquisition and organelle subversion by C. trachomatis. We show by live cell fluorescence microscopy and electron microscopy that lipid droplets (LDs), neutral lipid storage organelles, are translocated from the host cytoplasm into the inclusion lumen. LDs dock at the surface of the inclusion, penetrate the inclusion membrane and intimately associate with reticulate Bodies, the replicative form of Chlamydia. The inclusion membrane protein IncA, but not other inclusion membrane proteins, cofractionated with LDs and accumulated in the inclusion lumen. Therefore, we postulate that the translocation of LDs may occur at IncA-enriched subdomains of the inclusion membrane. Finally, the chlamydial protein Lda3 may participate in the cooption of these organelles by linking cytoplasmic LDs to inclusion membranes and promoting the removal of the LD protective coat protein, adipocyte differentiation related protein (ADRP). The wholesale transport of LDs into the lumen of a parasitophorous vacuole represents a unique mechanism of organelle sequestration and subversion by a bacterial pathogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schachter J. In: Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. Stephens RS, editor. Washington, D.C.: ASM; 1999. pp. 139–169.
-
- Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol. 2004;2:530–531. - PubMed
-
- Fields KA, Hackstadt T. The chlamydial inclusion: Escape from the endocytic pathway. Annu Rev Cell Dev Biol. 2002;18:221–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

