Is fetal brain monoamine oxidase inhibition the missing link between maternal smoking and conduct disorders?
- PMID: 18592036
- PMCID: PMC2441882
Is fetal brain monoamine oxidase inhibition the missing link between maternal smoking and conduct disorders?
Abstract
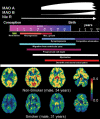
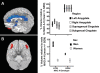
Smoking is the leading cause of preventable illness in the world today. Prenatal cigarette smoke exposure (PCSE) is a particularly insidious form because so many of its associated health effects befall the unborn child and produce behavioural outcomes that manifest themselves only years later. Among these are the associations between PCSE and conduct disorders, which have been mostly ascribed to the deleterious effects of nicotine on the fetal brain. Here we hypothesize that inhibition of brain monoamine oxidase (MAO) during fetal brain development, secondary to maternal cigarette smoking and in addition to nicotine, is a likely contributor to this association. MAOs play a central role in monoaminergic balance in the brain, and their inhibition during fetal development - but not during adult life - is known to result in an aggressive phenotype in laboratory animals. This paper provides theoretical and experimental support for the notion that cigarette smoke-induced inhibition of MAO in the fetal brain, particularly when it occurs in combination with polymorphisms in the MAOA gene that lead to lower enzyme concentration in the brain, may result in brain morphologic and functional changes that enhance the risk of irritability, poor self-control and aggression in the offspring. It also encourages research to evaluate whether the interaction of smoking exposure during fetal development and MAOA genotype increases the risk for conduct disorder over that incurred by mere fetal exposure to tobacco smoke.
Le tabagisme est maintenant la principale cause de maladies évitables dans le monde. L'exposition prénatale à la fumée de cigarette (EPFC) est particulièrement insidieuse parce que l'enfant à naître subit un très grand nombre des effets néfastes du tabagisme sur la santé et que cette atteinte produit des résultats comportementaux qui se manifestent seulement des années plus tard. Il y a notamment association entre l'EPFC et le trouble des conduites, que l'on a attribués pour la plupart aux effets nocifs de la nicotine sur le cerveau du fœtus. Nous posons ici en hypothèse que l'inhibition des monoamines oxydases (MAO) dans le cerveau au cours du développement du fœtus, résultat secondaire du tabagisme de la mère et qui s'ajoute à la dépendance à la nicotine, contribue probablement à ce lien. Les MAO jouent un rôle central dans l'équilibre monoaminergique du cerveau et on sait que leur inhibition au cours du développement du fœtus — mais non durant la vie adulte — produit un phénotype agressif chez des animaux de laboratoire. Cette communication appuie par la théorie et des données expérimentales le concept selon lequel l'inhibition des MAO provoquée par la fumée de cigarette dans le cerveau du fœtus, particulièrement lorsqu'elle est combinée à des polymorphismes du gène MAOA qui entraînent une baisse de la concentration d'enzymes dans le cerveau, peut produire dans celui-ci des changements morphologiques et fonctionnels qui favorisent le risque d'irritabilité, de manque de contrôle de soi et d'agression chez les enfants. Elle encourage aussi la recherche visant à évaluer si l'interaction entre l'exposition au tabagisme au cours du développement du fœtus et le génotype MAOA alourdit le risque de trouble des conduites par rapport au risque qui découle de la simple exposition du fœtus à la fumée de tabac.
Keywords: conduct disorder; maternal-fetal exchange; monoamine oxidase; smoking.
Figures


References
-
- Tobacco use among adults—United States, 2005. MMWR 2006;55;1145-8. - PubMed
-
- Levy DT, Nikolayev L, Mumford E, et al. The Healthy People 2010 smoking prevalence and tobacco control objectives: results from the SimSmoke tobacco control policy simulation model (United States). Cancer Causes Control 2005;16:359-71. - PubMed
-
- Centers for Disease Control and Prevention. Preventing chronic diseases: investing wisely in health [Chronic Disease Prevention factsheet]. Bethesda (MD): US Department of Health and Human Services; 2006.
-
- Jaddoe VW, Verburg BO, de Ridder MA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol 2007;165:1207-15. - PubMed
-
- Neuman RJ, Lobos E, Reich W, et al. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry 2006;61:1320-8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
