Stabilization of fully reduced iron-sulfur clusters by carbene ligation: the [FenSn]0 oxidation levels (n = 4, 8)
- PMID: 18593124
- PMCID: PMC2527442
- DOI: 10.1021/ja802111w
Stabilization of fully reduced iron-sulfur clusters by carbene ligation: the [FenSn]0 oxidation levels (n = 4, 8)
Abstract
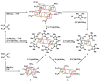
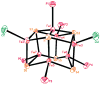
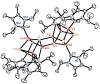
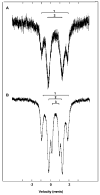
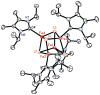
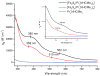
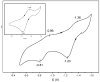
The all-ferrous [Fe4S4](0) state has been demonstrated in the fully reduced Fe protein of the Azotobacter vinelandii nitrogenase complex. We seek synthetic analogues of this state more tractable than the recently prepared but highly unstable cluster [Fe4S4(CN)4](4-) (Scott, Berlinguette, Holm, and Zhou, Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 9741). The N-heterocyclic carbene 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene (Pr(i)2NHCMe2) has been found to stabilize the fully reduced clusters [Fe8S8(Pr(i)2NHCMe2)6] (4) and [Fe4S4(Pr(i)2NHCMe2)4] (5), which are prepared by cluster assembly or phosphine substitution of FenSn (n = 8, 16) clusters. Cluster 4 is also obtained by reaction of the carbene with all-ferrous [Fe7S6(PEt3)5Cl2] (3) and cluster 5 by carbene cleavage of 4. Detailed structures of 3 (monocapped prismatic), 4, and 5 are described; the latter two are the first iron-sulfur clusters with Fe-C sigma bonds. Cluster 4 possesses the [Fe8(mu3-S) 6(mu4-S)2] edge-bridged double cubane structure and 5 the cubane-type [Fe4(mu3-S)4] stereochemistry. The all-ferrous formulations of the clusters are confirmed by X-ray structure parameters and (57)Fe isomer shifts. Both clusters are stable under conventional aprotic anaerobic conditions, enabling further study of reactivity. The collective properties of 5 indicate that it is a meaningful synthetic analogue of the core of the fully reduced protein-bound cluster.
Figures
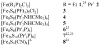
Similar articles
-
Mössbauer, electron paramagnetic resonance, and theoretical studies of a carbene-based all-ferrous Fe4S4 cluster: electronic origin and structural identification of the unique spectroscopic site.Inorg Chem. 2009 Apr 6;48(7):2735-47. doi: 10.1021/ic802192w. Inorg Chem. 2009. PMID: 19326927 Free PMC article.
-
Stabilization of reduced molybdenum-iron-sulfur single- and double-cubane clusters by cyanide ligation.Inorg Chem. 2007 Jan 22;46(2):510-6. doi: 10.1021/ic061704y. Inorg Chem. 2007. PMID: 17279830 Free PMC article.
-
Initial synthesis and structure of an all-ferrous analogue of the fully reduced [Fe4S4]0 cluster of the nitrogenase iron protein.Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9741-4. doi: 10.1073/pnas.0504258102. Epub 2005 Jun 28. Proc Natl Acad Sci U S A. 2005. PMID: 15985547 Free PMC article.
-
Iron-sulfur proteins: new roles for old clusters.Curr Opin Chem Biol. 1998 Apr;2(2):173-81. doi: 10.1016/s1367-5931(98)80058-6. Curr Opin Chem Biol. 1998. PMID: 9667933 Review.
-
Mössbauer spectroscopy of Fe/S proteins.Biochim Biophys Acta. 2015 Jun;1853(6):1395-405. doi: 10.1016/j.bbamcr.2014.12.005. Epub 2014 Dec 10. Biochim Biophys Acta. 2015. PMID: 25498248 Review.
Cited by
-
Specific incorporation of chalcogenide bridge atoms in molybdenum/tungsten-iron-sulfur single cubane clusters.Inorg Chem. 2011 Nov 7;50(21):11242-51. doi: 10.1021/ic2018117. Epub 2011 Oct 10. Inorg Chem. 2011. PMID: 21985054 Free PMC article.
-
Electrically conductive [Fe4S4]-based organometallic polymers.Chem Sci. 2023 Oct 4;14(41):11410-11416. doi: 10.1039/d3sc02195e. eCollection 2023 Oct 25. Chem Sci. 2023. PMID: 37886097 Free PMC article.
-
Stabilization of 3:1 site-differentiated cubane-type clusters in the [Fe(4)S(4)](1+) core oxidation state by tertiary phosphine ligation: synthesis, core structural diversity, and S = 1/2 ground states.Inorg Chem. 2010 Dec 6;49(23):11118-26. doi: 10.1021/ic101702b. Epub 2010 Nov 1. Inorg Chem. 2010. PMID: 21038882 Free PMC article.
-
Structural characterization of CO-inhibited Mo-nitrogenase by combined application of nuclear resonance vibrational spectroscopy, extended X-ray absorption fine structure, and density functional theory: new insights into the effects of CO binding and the role of the interstitial atom.J Am Chem Soc. 2014 Nov 12;136(45):15942-54. doi: 10.1021/ja505720m. Epub 2014 Nov 3. J Am Chem Soc. 2014. PMID: 25275608 Free PMC article.
-
Developments in the biomimetic chemistry of cubane-type and higher nuclearity iron-sulfur clusters.Chem Rev. 2014 Apr 9;114(7):3579-600. doi: 10.1021/cr4004067. Epub 2014 Jan 13. Chem Rev. 2014. PMID: 24410527 Free PMC article. Review. No abstract available.
References
-
- Beinert H, Holm RH, Münck E. Science. 1997;277:653–659. - PubMed
-
- Noodleman L, Lovell T, Liu T, Himo F, Torres RA. Curr Opin Chem Biol. 2002;6:259–273. - PubMed
-
- Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Howard JB, Rees DC. Chem Rev. 1996;96:2965–2982. - PubMed
-
- Watt GD, Reddy KRN. J Inorg Biochem. 1994;53:281–294.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

