Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells
- PMID: 18593336
- PMCID: PMC2702120
- DOI: 10.1089/scd.2008.0113
Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells
Abstract
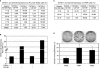
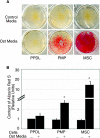
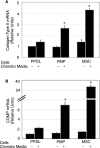
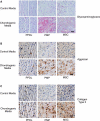
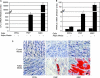
Although mesenchymal progenitor cells can be isolated from periodontal ligament (PDL) tissues using stem cell markers STRO-1 and CD146, the proportion of these cells that have the capacity to differentiate into multiple cell lineages remains to be determined. This study was designed to quantify the proportions of primary human PDL cells that can undergo multilineage differentiation and to compare the magnitude of these capabilities relative to bone marrow-derived mesenchymal stem cells (MSCs) and parental PDL (PPDL) cells. PDL mesenchymal progenitor (PMP) cells were isolated from PPDL cells using the markers STRO-1 and CD146. The colony-forming efficiency and multilineage differentiation potential of PMP, PPDL, and MSCs under chondrogenic, osteogenic, and adipogenic conditions were determined. Flow cytometry revealed that on average 2.6% of PPDL cells were STRO-1(+)/CD146(+), whereas more than 63% were STRO-1(-)/CD146(-). Colony-forming efficiency of STRO-1(+)/CD146(+) PMP cells (19.3%) and MSCs (16.7%) was significantly higher than that of PPDL cells (6.8%). Cartilage-specific genes, early markers of osteoblastic differentiation, and adipogenic markers were significantly upregulated under appropriate conditions in PMP cells and MSCs compared to either their noninduced counterparts or induced PPDL cells. Consistent with these findings, immunohistochemistry revealed substantial accumulation of cartilaginous macromolecules, mineralized calcium nodules, and lipid vacuoles under chondrogenic, osteogenic, or adipogenic conditions in PMP and MSC cultures, respectively, compared to noninduced controls or induced PPDL cells. Thus STRO-1(+)/CD146(+) PMP cells demonstrate multilineage differentiation capacity comparable in magnitude to MSCs and could potentially be utilized for regeneration of the periodontium and other tissues.
Figures
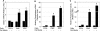
References
-
- Polimeni G. Xiropaidis AV. Wikesjo UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 2006;41:30–47. - PubMed
-
- Ivanovski S. Gronthos S. Shi S. Bartold PM. Stem cells in the periodontal ligament. Oral Dis. 2006;12:358–363. - PubMed
-
- Page RC. Offenbacher S. Schroeder HE. Seymour GJ. Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. - PubMed
-
- Ramakrishnan PR. Lin WL. Sodek J. Cho MI. Synthesis of noncollagenous extracellular matrix proteins during development of mineralized nodules by rat periodontal ligament cells in vitro. Calcif Tissue Int. 1995;57:52–59. - PubMed
-
- McCulloch CA. Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991;26(3 Pt 1):144–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

