Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs
- PMID: 18593559
- PMCID: PMC2497439
- DOI: 10.1016/j.stem.2008.04.004
Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs
Abstract
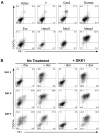
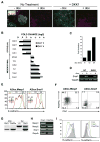
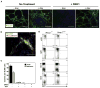
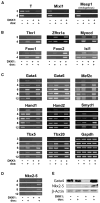
Wnt signaling is required for development of mesoderm-derived lineages and expression of transcription factors associated with the primitive streak. In a functional screen, we examined the mesoderm-inducing capacity of transcription factors whose expression was Wnt-dependent in differentiating ESCs. In contrast to many inactive factors, we found that mesoderm posterior 1 (Mesp1) promoted mesoderm development independently of Wnt signaling. Transient Mesp1 expression in ESCs promotes changes associated with epithelial-mesenchymal transition (EMT) and induction of Snai1, consistent with a role in gastrulation. Mesp1 expression also restricted the potential fates derived from ESCs, generating mesoderm progenitors with cardiovascular, but not hematopoietic, potential. Thus, in addition to its effects on EMT, Mesp1 may be capable of generating the recently identified multipotent cardiovascular progenitor from ESCs in vitro.
Figures


Comment in
-
Mesp1 at the heart of mesoderm lineage specification.Cell Stem Cell. 2008 Jul 3;3(1):1-2. doi: 10.1016/j.stem.2008.06.017. Cell Stem Cell. 2008. PMID: 18593549
References
-
- Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptor-alpha is a key determinant of smooth muscle alpha-actin filaments in bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol. 2007;39:379–391. - PubMed
-
- Cooper TA, Ordahl CP. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985;260:11140–11148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

