Islet oxygen consumption and insulin secretion tightly coupled to calcium derived from L-type calcium channels but not from the endoplasmic reticulum
- PMID: 18593707
- PMCID: PMC2528984
- DOI: 10.1074/jbc.M802097200
Islet oxygen consumption and insulin secretion tightly coupled to calcium derived from L-type calcium channels but not from the endoplasmic reticulum
Abstract
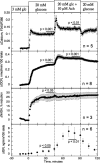
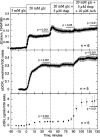
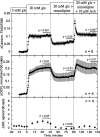
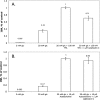
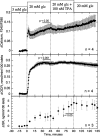
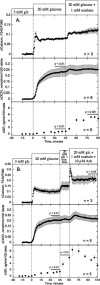
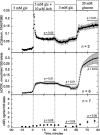
The aim of the study was to test whether the source of intracellular calcium (Ca2+) is a determinant of beta cell function. We hypothesized that elevations in cytosolic Ca2+ caused by the release of Ca2+ from the endoplasmic reticulum (ER) have little physiologic impact on oxygen consumption and insulin secretion. Ca2+ release from the ER was induced in isolated rat islets by acetylcholine and response of oxygen consumption rate (OCR), NAD(P)H, cytosolic Ca2+, and insulin secretory rate (ISR) were measured. Glucose increased all four parameters, and thereafter acetylcholine further increased cytosolic Ca2+, OCR, and ISR. To assess the contribution of Ca2+ release from the ER in mediating the effects of acetylcholine, ER Ca2+ stores were first emptied by inhibiting the sarcoendoplasmic reticulum Ca2+-ATPase, which subsequently reduced the effect of acetylcholine on cytosolic Ca2+ but not its effects on OCR or ISR. As predicted, OCR and ISR were acutely sensitive to changes in L-type Ca2+ channel activity; nimodipine completely inhibited glucose-stimulated ISR and suppressed OCR by 36%, despite only inhibiting cytosolic Ca2+ by 46%. Moreover, in the presence of nimodipine and high glucose, acetylcholine still elevated cytosolic Ca2+ levels above those observed in the presence of high glucose alone but did not significantly stimulate ISR. In conclusion, Ca2+ flux through L-type Ca2+ channels was tightly coupled to changes in OCR and ISR. In contrast, the results obtained support the notion that Ca2+ release from the ER has little or no access to the intracellular machinery that regulates OCR and ISR.
Figures

Similar articles
-
A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic beta-cells.Am J Physiol Endocrinol Metab. 2009 Sep;297(3):E717-27. doi: 10.1152/ajpendo.00282.2009. Epub 2009 Jul 7. Am J Physiol Endocrinol Metab. 2009. PMID: 19584201 Free PMC article.
-
Palmitate is not an effective fuel for pancreatic islets and amplifies insulin secretion independent of calcium release from endoplasmic reticulum.Islets. 2019;11(3):51-64. doi: 10.1080/19382014.2019.1601490. Epub 2019 May 14. Islets. 2019. PMID: 31084524 Free PMC article.
-
Contribution of calcium influx in mediating glucose-stimulated oxygen consumption in pancreatic islets.Diabetes. 2006 Dec;55(12):3509-19. doi: 10.2337/db06-0400. Diabetes. 2006. PMID: 17130499
-
Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans.Endocrine. 2000 Dec;13(3):251-62. doi: 10.1385/ENDO:13:3:251. Endocrine. 2000. PMID: 11216635 Review.
-
Regulation of Ca2+ homeostasis by islet endoplasmic reticulum and its role in insulin secretion.Am J Physiol. 1988 Feb;254(2 Pt 1):E121-36. doi: 10.1152/ajpendo.1988.254.2.E121. Am J Physiol. 1988. PMID: 3279798 Review.
Cited by
-
Reducing VEGFB expression regulates the balance of glucose and lipid metabolism in mice via VEGFR1.Mol Med Rep. 2022 Sep;26(3):285. doi: 10.3892/mmr.2022.12801. Epub 2022 Jul 27. Mol Med Rep. 2022. PMID: 35894135 Free PMC article.
-
The Impact of Calcium Overload on Cellular Processes: Exploring Calcicoptosis and Its Therapeutic Potential in Cancer.Int J Mol Sci. 2024 Dec 23;25(24):13727. doi: 10.3390/ijms252413727. Int J Mol Sci. 2024. PMID: 39769488 Free PMC article. Review.
-
Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics.Am J Physiol Endocrinol Metab. 2012 Jan 1;302(1):E87-E102. doi: 10.1152/ajpendo.00218.2011. Epub 2011 Sep 27. Am J Physiol Endocrinol Metab. 2012. PMID: 21952036 Free PMC article.
-
A method for high-throughput functional imaging of single cells within heterogeneous cell preparations.Sci Rep. 2016 Dec 16;6:39319. doi: 10.1038/srep39319. Sci Rep. 2016. PMID: 27982116 Free PMC article.
-
Hypoxia reduces HNF4α/MODY1 protein expression in pancreatic β-cells by activating AMP-activated protein kinase.J Biol Chem. 2017 May 26;292(21):8716-8728. doi: 10.1074/jbc.M116.767574. Epub 2017 Mar 31. J Biol Chem. 2017. PMID: 28364040 Free PMC article.
References
-
- Henquin, J. C., Ravier, M. A., Nenquin, M., Jonas, J. C., and Gilon, P. (2003) Eur. J. Clin. Investig. 33 742–750 - PubMed
-
- Satin, L. S. (2000) Endocrine 13 251–262 - PubMed
-
- Wollheim, C. B., and Sharp, G. W. (1981) Physiol. Rev. 61 914–973 - PubMed
-
- Prentki, M., and Matschinsky, F. M. (1987) Physiol. Rev. 67 1185–1248 - PubMed
-
- Arredouani, A., Henquin, J. C., and Gilon, P. (2002) Am. J. Physiol. 282 E982–E991 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

