Structural basis for the activation of FGFR by NCAM
- PMID: 18593816
- PMCID: PMC2548372
- DOI: 10.1110/ps.035964.108
Structural basis for the activation of FGFR by NCAM
Abstract
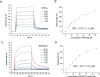
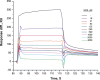
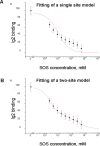
The fibroblast growth factor receptor (FGFR) can be activated through direct interaction with the neural cell adhesion molecule (NCAM). The extracellular part of the FGFR consists of three immunoglobulin-like (Ig) modules, and that of the NCAM consists of five Ig and two fibronectin type III (F3) modules. NCAM-FGFR interactions are mediated by the third FGFR Ig module and the second NCAM F3 module. Using surface plasmon resonance and nuclear magnetic resonance analyses, the present study demonstrates that the second Ig module of FGFR also is involved in binding to the NCAM. The second Ig module residues involved in binding were identified and shown to be localized on the "opposite sides" of the module, indicating that when NCAMs are clustered (e.g., due to homophilic binding), high-affinity FGFR binding sites may be formed by the neighboring NCAMs.
Figures

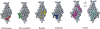
Similar articles
-
Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP.Structure. 2003 Jun;11(6):691-701. doi: 10.1016/s0969-2126(03)00096-0. Structure. 2003. PMID: 12791257
-
NCAM-derived peptides function as agonists for the fibroblast growth factor receptor.J Neurochem. 2008 Sep;106(5):2030-41. doi: 10.1111/j.1471-4159.2008.05544.x. Epub 2008 Jul 4. J Neurochem. 2008. PMID: 18624916
-
A peptide motif from the second fibronectin module of the neural cell adhesion molecule, NCAM, NLIKQDDGGSPIRHY, is a binding site for the FGF receptor.Neurochem Res. 2008 Dec;33(12):2532-9. doi: 10.1007/s11064-008-9680-2. Epub 2008 Mar 27. Neurochem Res. 2008. PMID: 18368482
-
Structural biology of NCAM homophilic binding and activation of FGFR.J Neurochem. 2005 Sep;94(5):1169-79. doi: 10.1111/j.1471-4159.2005.03284.x. Epub 2005 Jul 25. J Neurochem. 2005. PMID: 16045455 Review.
-
Neuritogenic and neuroprotective properties of peptide agonists of the fibroblast growth factor receptor.Int J Mol Sci. 2010 May 26;11(6):2291-305. doi: 10.3390/ijms11062291. Int J Mol Sci. 2010. PMID: 20640153 Free PMC article. Review.
Cited by
-
Variable Expression of Neural Cell Adhesion Molecule Isoforms in Renal Tissue: Possible Role in Incipient Renal Fibrosis.PLoS One. 2015 Sep 1;10(9):e0137028. doi: 10.1371/journal.pone.0137028. eCollection 2015. PLoS One. 2015. PMID: 26327314 Free PMC article.
-
Cell Proliferation and Collective Cell Migration During Zebrafish Lateral Line System Development Are Regulated by Ncam/Fgf-Receptor Interactions.Front Cell Dev Biol. 2021 Jan 14;8:591011. doi: 10.3389/fcell.2020.591011. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33520983 Free PMC article.
-
NCAM1/FGF module serves as a putative pleuropulmonary blastoma therapeutic target.Oncogenesis. 2019 Sep 2;8(9):48. doi: 10.1038/s41389-019-0156-9. Oncogenesis. 2019. PMID: 31477684 Free PMC article.
-
Cell adhesion and intracellular calcium signaling in neurons.Cell Commun Signal. 2013 Dec 13;11:94. doi: 10.1186/1478-811X-11-94. Cell Commun Signal. 2013. PMID: 24330678 Free PMC article.
-
NCAM and FGFR1 coexpression and colocalization in renal tumors.Int J Clin Exp Pathol. 2014 Mar 15;7(4):1402-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24817936 Free PMC article.
References
-
- Atkins, A.R., Osborne, M.J., Lashuel, H.A., Edelman, G.M., Wright, P.E., Cunningham, B.A., Dyson, H.J. Association between the first two immunoglobulin-like domains of the neural cell adhesion molecule N-CAM. FEBS Lett. 1999;451:162–168. - PubMed
-
- Berezin, V., Bock, E., Poulsen, F.M. The neural cell adhesion molecule. Curr. Opin. Drug Discov. Dev. 2000;3:605–609. - PubMed
-
- Bruses, J.L., Rutishauser, U. Roles, regulation, and mechanism of polysialic acid function during neural development. Biochimie. 2001;83:635–643. - PubMed
-
- Cremer, H., Chazal, G., Goridis, C., Represa, A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell. Neurosci. 1997;8:323–335. - PubMed
-
- Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biol. NMR. 1995;6:277–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

