Haploinsufficiency of the maspin tumor suppressor gene leads to hyperplastic lesions in prostate
- PMID: 18593913
- PMCID: PMC2518316
- DOI: 10.1158/0008-5472.CAN-08-0163
Haploinsufficiency of the maspin tumor suppressor gene leads to hyperplastic lesions in prostate
Abstract
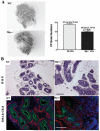
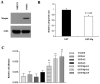
Maspin is a key tumor suppressor gene in prostate and breast cancers with diverse biological functions. However, how maspin regulates prostate tumor progression is not fully understood. In this study, we have used maspin heterozygous knockout mice to determine the effect of maspin haploinsufficiency on prostate development and tumor progression. We report that loss of one copy of maspin gene in Mp(+/-) heterozygous knockout mice leads to the development of prostate hyperplastic lesions, and this effect was mediated through decreased level of cyclin-dependent kinase inhibitors p21 and p27. Prostate hyperplastic lesions in Mp(+/-) mice also induced stromal reaction, which occurred in both aged prostate tissues and in neonatal prostates during early ductal morphogenesis. We showed that maspin was also expressed in prostate smooth muscle cells (PSMC), and recombinant maspin increased PSMC cell adhesion but inhibited cell proliferation. We also observed a defective interaction between epithelial cells and basement membrane in the prostate of Mp(+/-) mice, which was accompanied with a changed pattern of matrix deposition and a loss of epithelial cell polarity. Therefore, we have identified a novel property of maspin, which involves the control of the proliferation in prostate epithelial and smooth muscle cells. This is the first report that a partial loss of maspin caused an early developmental defect of the prostate and prostate hyperplastic lesions in mouse.
Figures




Similar articles
-
Maspin plays an essential role in early embryonic development.Development. 2004 Apr;131(7):1479-89. doi: 10.1242/dev.01048. Epub 2004 Feb 25. Development. 2004. PMID: 14985257
-
Maspin functions as tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells.J Urol. 2003 Mar;169(3):1157-61. doi: 10.1097/01.ju.0000040245.70349.37. J Urol. 2003. PMID: 12576872
-
The secretion and biological function of tumor suppressor maspin as an exosome cargo protein.Oncotarget. 2017 Jan 31;8(5):8043-8056. doi: 10.18632/oncotarget.13302. Oncotarget. 2017. PMID: 28009978 Free PMC article.
-
Tumor suppressor maspin as a rheostat in HDAC regulation to achieve the fine-tuning of epithelial homeostasis.Crit Rev Eukaryot Gene Expr. 2012;22(3):249-58. doi: 10.1615/critreveukargeneexpr.v22.i3.80. Crit Rev Eukaryot Gene Expr. 2012. PMID: 23140166 Free PMC article. Review.
-
Multiple functions of maspin in tumor progression and mouse development.Front Biosci. 2004 Sep 1;9:2218-26. doi: 10.2741/1392. Front Biosci. 2004. PMID: 15353283 Review.
Cited by
-
G-helix of maspin mediates effects on cell migration and adhesion.J Biol Chem. 2010 Nov 19;285(47):36285-92. doi: 10.1074/jbc.M110.177253. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837467 Free PMC article.
-
Haploinsufficiency of Tumor Suppressor Genes is Driven by the Cumulative Effect of microRNAs, microRNA Binding Site Polymorphisms and microRNA Polymorphisms: An In silico Approach.Cancer Inform. 2012;11:157-71. doi: 10.4137/CIN.S10176. Epub 2012 Aug 29. Cancer Inform. 2012. PMID: 23032637 Free PMC article.
-
The roles of maspin expression in gastric cancer: a meta- and bioinformatics analysis.Oncotarget. 2017 Aug 11;8(39):66476-66490. doi: 10.18632/oncotarget.20192. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029529 Free PMC article.
-
Association between SNPs in Serpin gene family and risk of esophageal squamous cell carcinoma.Tumour Biol. 2015 Aug;36(8):6231-8. doi: 10.1007/s13277-015-3308-3. Epub 2015 Mar 17. Tumour Biol. 2015. PMID: 25775950
-
SiO2 nanoparticles induce cytotoxicity and protein expression alteration in HaCaT cells.Part Fibre Toxicol. 2010 Jan 19;7:1. doi: 10.1186/1743-8977-7-1. Part Fibre Toxicol. 2010. PMID: 20180970 Free PMC article.
References
-
- Pemberton PA, Tipton AR, Pavloff N, et al. Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem. 1997;45:1697–706. - PubMed
-
- Shi HY, Zhang W, Liang R, et al. Blocking tumor growth, invasion, and metastasis by maspin in a syngeneic breast cancer model. Cancer research. 2001;61:6945–51. - PubMed
-
- Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

