Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody
- PMID: 18593929
- PMCID: PMC2585743
- DOI: 10.1158/0008-5472.CAN-08-0037
Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody
Abstract
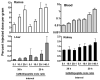
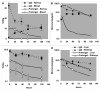
We examined whether a pretargeting method using a new recombinant anti-CD20 bispecific antibody (bsMAb) followed by (90)Y-1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid ((90)Y-DOTA)-peptide could reduce hematologic toxicity yet improve therapeutic responses compared with conventional (90)Y-anti-CD20 IgG and a chemically conjugated bsMAb. TF4, a humanized, tri-Fab bsMAb with two Fabs binding CD20 and one Fab binding histamine-succinyl-glycine (HSG), developed by the dock and lock (DNL) method, was tested in nude mice with Ramos B-cell lymphomas. Optimal pretargeting required a 29-h interval between TF4 and (90)Y-DOTA-HSG, and 20-fold more moles of TF4. TF4 cleared more rapidly from the blood than anti-CD20 IgG, with early processing in the liver, spleen, and kidney. At 24 h, TF4 improved tumor uptake of (111)In-HSG-peptide 2.6-fold [13% versus 5% injected dose per gram (ID/g)] and enhanced tumor to blood ratios >45-fold (770 versus 17), compared with an anti-CD20 Fab x anti-HSG Fab chemical conjugate, and by 1.6-fold (9.0% versus 5.6% ID/g) and 1,600-fold (522 versus 0.32), respectively, compared with radiolabeled anti-CD20 IgG. A severe (>or=90%) and prolonged reduction of WBCs was observed at the maximum dose of (90)Y-anti-CD20 IgG, whereas pretargeting resulted in a <or=60% transient drop. TF4 pretargeting resulted in highly significant improvement in survival, curing 33% to 90% of the animals, even at relatively low doses, whereas most tumors progressed quickly without cures with (90)Y-anti-CD20 IgG. These results indicate an improved therapeutic index with pretargeted radioimmunotherapy (RAIT) using a DNL-constructed tri-Fab, bsMAb, compared with conventional therapy with directly radiolabeled antibody or with a chemically conjugated bsMAb. These encouraging results prompt testing these constructs for pretargeting RAIT in patients.
Figures


Similar articles
-
Improved therapy of non-Hodgkin's lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody.Leukemia. 2005 Jun;19(6):1064-9. doi: 10.1038/sj.leu.2403751. Leukemia. 2005. PMID: 15815716
-
Optimizing bispecific antibody pretargeting for use in radioimmunotherapy.Clin Cancer Res. 2003 Sep 1;9(10 Pt 2):3897S-913S. Clin Cancer Res. 2003. PMID: 14506188
-
Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody's valency for the tumor target antigen.Bioconjug Chem. 2002 Sep-Oct;13(5):1054-70. doi: 10.1021/bc0200172. Bioconjug Chem. 2002. PMID: 12236788
-
111In-Labeled 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid (DOTA)-d-Tyr-d-Lys(HSG)-d-Glu-d-Lys(HSG)-NH2 (IMP-288).2011 Jun 29 [updated 2011 Aug 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jun 29 [updated 2011 Aug 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21882402 Free Books & Documents. Review.
-
Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy.Semin Nucl Med. 2010 May;40(3):190-203. doi: 10.1053/j.semnuclmed.2009.12.002. Semin Nucl Med. 2010. PMID: 20350628 Free PMC article. Review.
Cited by
-
Pretargeted molecular imaging and radioimmunotherapy.Theranostics. 2012;2(5):523-40. doi: 10.7150/thno.3582. Epub 2012 May 17. Theranostics. 2012. PMID: 22737190 Free PMC article.
-
A modular IgG-scFv bispecific antibody topology.Protein Eng Des Sel. 2010 Apr;23(4):221-8. doi: 10.1093/protein/gzp077. Epub 2009 Dec 17. Protein Eng Des Sel. 2010. PMID: 20019028 Free PMC article.
-
Therapeutic Applications of Pretargeting.Pharmaceutics. 2019 Sep 1;11(9):434. doi: 10.3390/pharmaceutics11090434. Pharmaceutics. 2019. PMID: 31480515 Free PMC article. Review.
-
Rapid detection of hypoxia-inducible factor-1-active tumours: pretargeted imaging with a protein degrading in a mechanism similar to hypoxia-inducible factor-1alpha.Eur J Nucl Med Mol Imaging. 2010 Aug;37(8):1566-74. doi: 10.1007/s00259-010-1467-4. Epub 2010 Apr 29. Eur J Nucl Med Mol Imaging. 2010. PMID: 20428865
-
A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: prospects for dual-targeted antibody/radioantibody therapy.Blood. 2009 Apr 23;113(17):3891-5. doi: 10.1182/blood-2008-11-188896. Epub 2009 Jan 30. Blood. 2009. PMID: 19182204 Free PMC article.
References
-
- Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006;19:621–8. - PubMed
-
- Cheson BD. Monoclonal antibody therapy for B-cell malignancies. Semin Oncol. 2006;33:S2–14. - PubMed
-
- Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:20–7. - PubMed
-
- Jacobs SA, Foon KA. The expanding role of rituximab and radioimmunotherapy in the treatment of B-cell lymphomas. Expert Opin Biol Ther. 2007;7:1749–62. - PubMed
-
- Martin P, Leonard JP. Targeted therapies for non-Hodgkin lymphoma: rationally designed combinations. Clin Lymphoma Myeloma. 2007;7 5:S192–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

