Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance
- PMID: 18593950
- PMCID: PMC2663383
- DOI: 10.1158/0008-5472.CAN-08-0594
Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance
Abstract
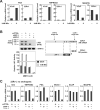
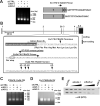
The standard systemic treatment for prostate cancer (PCa) is androgen ablation, which causes tumor regression by inhibiting activity of the androgen receptor (AR). Invariably, PCa recurs with a fatal androgen-refractory phenotype. Importantly, the growth of androgen-refractory PCa remains dependent on the AR through various mechanisms of aberrant AR activation. Here, we studied the 22Rv1 PCa cell line, which was derived from a CWR22 xenograft that relapsed during androgen ablation. Three AR isoforms are expressed in 22Rv1 cells: a full-length version with duplicated exon 3 and two truncated versions lacking the COOH terminal domain (CTD). We found that CTD-truncated AR isoforms are encoded by mRNAs that have a novel exon 2b at their 3' end. Functionally, these AR isoforms are constitutively active and promote the expression of endogenous AR-dependent genes, as well as the proliferation of 22Rv1 cells in a ligand-independent manner. AR mRNAs containing exon 2b and their protein products are expressed in commonly studied PCa cell lines. Moreover, exon 2b-derived species are enriched in xenograft-based models of therapy-resistant PCa. Together, our data describe a simple and effective mechanism by which PCa cells can synthesize a constitutively active AR and thus circumvent androgen ablation.
Figures




References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–244. - PubMed
-
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. - PubMed
-
- Matias PM, Donner P, Coelho R, et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J Biol Chem. 2000;275:26164–26171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

