Combined inhibition of PLC{gamma}-1 and c-Src abrogates epidermal growth factor receptor-mediated head and neck squamous cell carcinoma invasion
- PMID: 18594017
- PMCID: PMC3358699
- DOI: 10.1158/1078-0432.CCR-07-4857
Combined inhibition of PLC{gamma}-1 and c-Src abrogates epidermal growth factor receptor-mediated head and neck squamous cell carcinoma invasion
Abstract
Purpose: Mortality from head and neck squamous cell carcinoma (HNSCC) is usually associated with locoregional invasion of the tumor into vital organs, including the airway. Understanding the signaling mechanisms that abrogate HNSCC invasion may reveal novel therapeutic targets for intervention. The purpose of this study was to investigate the efficacy of combined inhibition of c-Src and PLCgamma-1 in the abrogation of HNSCC invasion.
Experimental design: PLCgamma-1 and c-Src inhibition was achieved by a combination of small molecule inhibitors and dominant negative approaches. The effect of inhibition of PLCgamma-1 and c-Src on invasion of HNSCC cells was assessed in an in vitro Matrigel-coated transwell invasion assay. In addition, the immunoprecipitation reactions and in silico database mining was used to examine the interactions between PLCgamma-1 and c-Src.
Results: Here, we show that inhibition of PLCgamma-1 or c-Src with the PLC inhibitor U73122 or the Src family inhibitor AZD0530 or using dominant-negative constructs attenuated epidermal growth factor (EGF)-stimulated HNSCC invasion. Furthermore, EGF stimulation increased the association between PLCgamma-1 and c-Src in HNSCC cells. Combined inhibition of PLCgamma-1 and c-Src resulted in further attenuation of HNSCC cell invasion in vitro.
Conclusions: These cumulative results suggest that PLCgamma-1 and c-Src activation contribute to HNSCC invasion downstream of EGF receptor and that targeting these pathways may be a novel strategy to prevent tumor invasion in HNSCC.
Figures

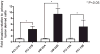
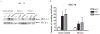


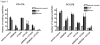
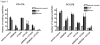

Similar articles
-
Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma.Clin Cancer Res. 2008 Jul 1;14(13):4284-91. doi: 10.1158/1078-0432.CCR-07-5226. Clin Cancer Res. 2008. PMID: 18594011 Free PMC article.
-
Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma.Cancer Res. 2003 Sep 1;63(17):5629-35. Cancer Res. 2003. PMID: 14500405
-
Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop.Oncogene. 2013 Oct;32(40):4766-77. doi: 10.1038/onc.2012.513. Epub 2012 Nov 12. Oncogene. 2013. PMID: 23146907 Free PMC article.
-
Molecular targeted therapies in the management of head and neck squamous cell carcinoma: recent developments and perspectives.Anticancer Agents Med Chem. 2013 Mar;13(3):389-402. Anticancer Agents Med Chem. 2013. PMID: 23092267 Review.
-
Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer.Mol Cancer. 2022 Jan 26;21(1):31. doi: 10.1186/s12943-022-01503-1. Mol Cancer. 2022. PMID: 35081970 Free PMC article. Review.
Cited by
-
Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation.J Biol Chem. 2011 Apr 29;286(17):15268-77. doi: 10.1074/jbc.M110.211813. Epub 2011 Jan 18. J Biol Chem. 2011. PMID: 21245141 Free PMC article.
-
Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion.Cancers (Basel). 2015 Feb 27;7(1):382-406. doi: 10.3390/cancers7010382. Cancers (Basel). 2015. PMID: 25734659 Free PMC article. Review.
-
Targeting cellular and molecular drivers of head and neck squamous cell carcinoma: current options and emerging perspectives.Cancer Metastasis Rev. 2016 Sep;35(3):413-26. doi: 10.1007/s10555-016-9625-1. Cancer Metastasis Rev. 2016. PMID: 27194534 Free PMC article. Review.
-
Mechanisms of Invasion in Head and Neck Cancer.Arch Pathol Lab Med. 2015 Nov;139(11):1334-48. doi: 10.5858/arpa.2014-0498-RA. Epub 2015 Jun 5. Arch Pathol Lab Med. 2015. PMID: 26046491 Free PMC article. Review.
-
Activation of alternate prosurvival pathways accounts for acquired sunitinib resistance in U87MG glioma xenografts.J Pharmacol Exp Ther. 2012 Nov;343(2):509-19. doi: 10.1124/jpet.112.196097. Epub 2012 Aug 6. J Pharmacol Exp Ther. 2012. PMID: 22869928 Free PMC article.
References
-
- Thomas SM, Coppelli FM, Wells A, et al. Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 2003;63:5629–35. - PubMed
-
- Kassis J, Moellinger J, Lo H, et al. A role for phospholipase C-gamma-mediated signaling in tumor cell invasion. Clin Cancer Res. 1999;5:2251–60. - PubMed
-
- Lotz M, Wang HH, Cance W, Matthews J, Pories S. Epidermal growth factor stimulation can substitute for c-Src overexpression in promoting breast carcinoma invasion. J Surg Res. 2003;109:123–9. - PubMed
-
- Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–56. - PubMed
-
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

