Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis
- PMID: 18594509
- PMCID: PMC2750091
- DOI: 10.1038/nature07091
Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis
Erratum in
-
Corrigendum: Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis.Nature. 2016 May 12;533(7602):278. doi: 10.1038/nature16543. Epub 2016 Jan 27. Nature. 2016. PMID: 26814969 No abstract available.
Expression of concern in
-
Editorial Expression of Concern: Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis.Nature. 2025 May;641(8062):E6. doi: 10.1038/s41586-025-09026-7. Nature. 2025. PMID: 40275109 No abstract available.
Abstract
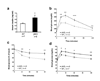
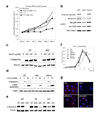
On activation by receptors, the ubiquitously expressed class IA isoforms (p110alpha and p110beta) of phosphatidylinositol-3-OH kinase (PI(3)K) generate lipid second messengers, which initiate multiple signal transduction cascades. Recent studies have demonstrated specific functions for p110alpha in growth factor and insulin signalling. To probe for distinct functions of p110beta, we constructed conditional knockout mice. Here we show that ablation of p110beta in the livers of the resulting mice leads to impaired insulin sensitivity and glucose homeostasis, while having little effect on phosphorylation of Akt, suggesting the involvement of a kinase-independent role of p110beta in insulin metabolic action. Using established mouse embryonic fibroblasts, we found that removal of p110beta also had little effect on Akt phosphorylation in response to stimulation by insulin and epidermal growth factor, but resulted in retarded cell proliferation. Reconstitution of p110beta-null cells with a wild-type or kinase-dead allele of p110beta demonstrated that p110beta possesses kinase-independent functions in regulating cell proliferation and trafficking. However, the kinase activity of p110beta was required for G-protein-coupled receptor signalling triggered by lysophosphatidic acid and had a function in oncogenic transformation. Most strikingly, in an animal model of prostate tumour formation induced by Pten loss, ablation of p110beta (also known as Pik3cb), but not that of p110alpha (also known as Pik3ca), impeded tumorigenesis with a concomitant diminution of Akt phosphorylation. Taken together, our findings demonstrate both kinase-dependent and kinase-independent functions for p110beta, and strongly indicate the kinase-dependent functions of p110beta as a promising target in cancer therapy.
Figures

References
-
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. - PubMed
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. - PubMed
-
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Liu Z, Roberts TM. Human tumor mutants in the p110alpha subunit of PI3K. Cell Cycle. 2006;5:675–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

