In vitro sensitivity testing of minimally passaged and uncultured gliomas with TRAIL and/or chemotherapy drugs
- PMID: 18594532
- PMCID: PMC2480982
- DOI: 10.1038/sj.bjc.6604459
In vitro sensitivity testing of minimally passaged and uncultured gliomas with TRAIL and/or chemotherapy drugs
Erratum in
- Br J Cancer. 2009 Mar 24;100(6):1012
Abstract
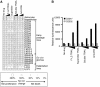
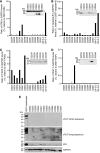
TRAIL/Apo-2L has shown promise as an anti-glioma drug, based on investigations of TRAIL sensitivity in established glioma cell lines, but it is not known how accurately TRAIL signalling pathways of glioma cells in vivo are reproduced in these cell lines in vitro. To replicate as closely as possible the in vivo behaviour of malignant glioma cells, 17 early passage glioma cell lines and 5 freshly resected gliomas were exposed to TRAIL-based agents and/or chemotherapeutic drugs. Normal human hepatocytes and astrocytes and established glioma cell lines were also tested. Cross-linked TRAIL, but not soluble TRAIL, killed both normal cell types and cells from three tumours. Cells from only one glioma were killed by soluble TRAIL, although only inefficiently. High concentrations of cisplatin were lethal to glioma cells, hepatocytes and astrocytes. Isolated combinations of TRAIL and chemotherapy drugs were more toxic to particular gliomas than normal cells, but no combination was generally selective for glioma cells. This study highlights the widespread resistance of glioma cells to TRAIL-based agents, but suggests that a minority of high-grade glioma patients may benefit from particular combinations of TRAIL and chemotherapy drugs. In vitro sensitivity assays may help identify effective drug combinations for individual glioma patients.
Figures





Similar articles
-
Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model.Cancer Res. 2004 Oct 1;64(19):6858-62. doi: 10.1158/0008-5472.CAN-04-1683. Cancer Res. 2004. PMID: 15466173
-
Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo.Cancer Res. 2003 Sep 1;63(17):5390-400. Cancer Res. 2003. PMID: 14500373
-
Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells.Gynecol Oncol. 2001 Jun;81(3):380-90. doi: 10.1006/gyno.2001.6194. Gynecol Oncol. 2001. PMID: 11371126
-
Adults with newly diagnosed high-grade gliomas.Curr Treat Options Oncol. 2001 Dec;2(6):507-15. doi: 10.1007/s11864-001-0072-y. Curr Treat Options Oncol. 2001. PMID: 12057096 Review.
-
Selectivity of TRAIL-mediated apoptosis of cancer cells and synergy with drugs: the trail to non-toxic cancer therapeutics (review).Int J Oncol. 1999 Oct;15(4):793-802. doi: 10.3892/ijo.15.4.793. Int J Oncol. 1999. PMID: 10493964 Review.
Cited by
-
A novel PTEN-dependent link to ubiquitination controls FLIPS stability and TRAIL sensitivity in glioblastoma multiforme.Cancer Res. 2009 Oct 15;69(20):7911-6. doi: 10.1158/0008-5472.CAN-09-1287. Epub 2009 Oct 6. Cancer Res. 2009. PMID: 19808964 Free PMC article.
-
TRAIL treatment provokes mutations in surviving cells.Oncogene. 2010 Sep 9;29(36):5048-60. doi: 10.1038/onc.2010.242. Epub 2010 Jul 19. Oncogene. 2010. PMID: 20639907 Free PMC article.
-
Low-dose anisomycin sensitizes melanoma cells to TRAIL induced apoptosis.Cancer Biol Ther. 2013 Feb;14(2):146-54. doi: 10.4161/cbt.22953. Epub 2012 Nov 28. Cancer Biol Ther. 2013. PMID: 23192275 Free PMC article.
-
Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors.J Natl Cancer Inst. 2014 Jul 30;106(8):dju186. doi: 10.1093/jnci/dju186. Print 2014 Aug. J Natl Cancer Inst. 2014. PMID: 25080574 Free PMC article. Review.
-
FOLFIRI plus dulanermin (rhApo2L/TRAIL) in a patient with BRAF-mutant metastatic colon cancer.Cancer Biol Ther. 2013 Aug;14(8):711-9. doi: 10.4161/cbt.25310. Epub 2013 Jun 13. Cancer Biol Ther. 2013. PMID: 23792567 Free PMC article.
References
-
- Anderson RC, Elder JB, Brown MD, Mandigo CE, Parsa AT, Kim PD, Senatus P, Anderson DE, Bruce JN (2002) Changes in the immunologic phenotype of human malignant glioma cells after passaging in vitro. Clin Immunol 102: 84–95 - PubMed
-
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104: 155–162 - PMC - PubMed
-
- Ashley DM, Riffkin CD, Muscat AM, Knight MJ, Kaye AH, Novak U, Hawkins CJ (2005) Caspase-8 is absent or low in many ex vivo gliomas. Cancer 104: 1487–1496 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

