Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats
- PMID: 18594556
- PMCID: PMC2587070
- DOI: 10.1038/jcbfm.2008.68
Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats
Abstract
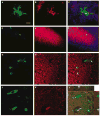
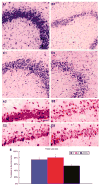
Once hypoxic-ischemic (HI) injury ensues in the human neonate at birth, the resulting brain damage lasts throughout the individual's lifetime, as no ameliorative treatments are currently available. We have recently shown that intracerebral transplantation of multipotent adult progenitor cells (MAPCs) results in behavioral improvement and reduction in ischemic cell loss in neonatal rat HI-injury model. In an attempt to advance this cellular therapy to the clinic, we explored the more practical and less invasive intravenous administration of MAPCs. Seven-day-old Sprague-Dawley rats were initially subjected to unilateral HI injury, then 7 days later received intracerebral or intravenous injections of allogeneic rat MAPCs. On post-transplantation days 7 and 14, the animals that received MAPCs via the intracerebral or intravenous route exhibited improved motor and neurologic scores compared with those that received vehicle infusion alone. Immunohistochemical evaluations at day 14 after transplantation revealed that both intracerebrally and intravenously transplanted MAPCs were detected in the ischemic hippocampal area. The degree of hippocampal cell preservation was almost the same in the two treatment groups and greater than that in the vehicle group. These results show that intravenous delivery of MAPCs is a feasible and efficacious cell therapy with potential for clinical use.
Figures

References
-
- Borlongan CV, Evans A, Yu G, Hess DC. Limitations of intravenous human bone marrow CD133+ cell grafts in stroke rats. Brain Res. 2005;1048:116–22. - PubMed
-
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuro-protection in stroke. Stroke. 2004;35:2385–9. - PubMed
-
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. NeuroReport. 1998;9:3703–9. - PubMed
-
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemic injury in rats. Stroke. 2001;32:1005–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

