Postnatal expression of corticotropin releasing factor (CRF) in rat urinary bladder
- PMID: 18595780
- PMCID: PMC2569849
- DOI: 10.1016/j.autneu.2008.05.007
Postnatal expression of corticotropin releasing factor (CRF) in rat urinary bladder
Abstract
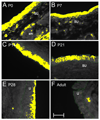
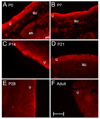
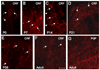
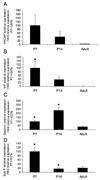
Corticotropin releasing factor (CRF) is a neuropeptide expressed in micturition reflex circuitry and different roles in these reflexes have been suggested. These studies examined the expression of CRF/CRF receptors in the urinary bladder during postnatal development in the rat. Urinary bladder was harvested from rats (postnatal (P) day 0-adult) euthanized by isoflurane (4%) and thoracotomy. CRF protein expression significantly (p<or=0.01) decreased in the urothelium with increasing postnatal age. In contrast, CRF-immunoreactivity (IR) was increased in nerve fibers in the suburothelial plexus during the second-third postnatal week. Total CRF protein from urinary bladder significantly increased during the second-third postnatal weeks as determined with ELISAs. CRF receptor 2 (CRFR(2)) transcript was expressed in urinary bladder of all postnatal ages examined whereas no CRFR(1) transcript was expressed at any postnatal age examined. We also demonstrated changes in urinary bladder mRNA expression for the neuropeptides, galanin, substance P, vasoactive intestinal polypeptide and pituitary adenylate cyclase activating polypeptide during postnatal development. These studies demonstrate changes in the CRF expression in urinary bladder, specifically in the urothelium and nerve fibers of the suburothelial plexus during postnatal development. Changes in CRF expression and neuropeptide expression in general in the urinary bladder may contribute to the emergence of mature voiding reflexes.
Figures




Similar articles
-
Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis.Am J Physiol Regul Integr Comp Physiol. 2006 Sep;291(3):R692-703. doi: 10.1152/ajpregu.00086.2006. Epub 2006 Apr 13. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16614059
-
Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium.J Mol Neurosci. 2012 Nov;48(3):730-43. doi: 10.1007/s12031-012-9834-1. Epub 2012 Jun 15. J Mol Neurosci. 2012. PMID: 22700375 Free PMC article.
-
Accelerated onset of the vesicovesical reflex in postnatal NGF-OE mice and the role of neuropeptides.Exp Neurol. 2016 Nov;285(Pt B):110-125. doi: 10.1016/j.expneurol.2016.06.021. Epub 2016 Jun 21. Exp Neurol. 2016. PMID: 27342083 Free PMC article.
-
Neuropeptides in lower urinary tract function.Handb Exp Pharmacol. 2011;(202):395-423. doi: 10.1007/978-3-642-16499-6_19. Handb Exp Pharmacol. 2011. PMID: 21290237 Free PMC article. Review.
-
The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract.J Mol Neurosci. 2008 Nov;36(1-3):227-40. doi: 10.1007/s12031-008-9090-6. Epub 2008 Aug 2. J Mol Neurosci. 2008. PMID: 18677446 Free PMC article. Review.
Cited by
-
The bladder-brain connection: putative role of corticotropin-releasing factor.Nat Rev Urol. 2011 Jan;8(1):19-28. doi: 10.1038/nrurol.2010.203. Epub 2010 Dec 7. Nat Rev Urol. 2011. PMID: 21135878 Free PMC article. Review.
-
Spinal CRH facilitates the micturition reflex via the CRH2 receptor in rats with normal bladder and bladder outlet obstruction.Sci Rep. 2025 Jan 29;15(1):3604. doi: 10.1038/s41598-025-87990-w. Sci Rep. 2025. PMID: 39875474 Free PMC article.
-
Neonatal cystitis leads to alterations in spinal corticotropin releasing factor receptor-type 2 content and function in adult rats following bladder re-inflammation.Brain Res. 2022 Aug 1;1788:147927. doi: 10.1016/j.brainres.2022.147927. Epub 2022 Apr 26. Brain Res. 2022. PMID: 35477003 Free PMC article.
-
Sound-stress-induced altered nociceptive behaviors are associated with increased spinal CRFR2 gene expression in a rat model of burn injury.J Pain Res. 2017 Sep 1;10:2135-2145. doi: 10.2147/JPR.S144055. eCollection 2017. J Pain Res. 2017. PMID: 28979159 Free PMC article.
References
-
- Benarroch EE, Schmeiche IAM. Depletion of corticotrophin-releasing factor neurons in the pontine micturition area in multiple system atrophy. Annals Neurol. 2001;50(5):640–645. - PubMed
-
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289(3):F489–F495. - PubMed
-
- Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol. 2006;45(4):221–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

