NK314, a topoisomerase II inhibitor that specifically targets the alpha isoform
- PMID: 18596031
- PMCID: PMC3259784
- DOI: 10.1074/jbc.M803936200
NK314, a topoisomerase II inhibitor that specifically targets the alpha isoform
Abstract
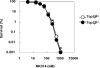
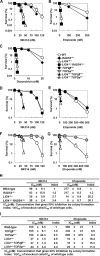
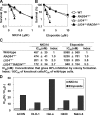
Topoisomerase II (Top2) is a ubiquitous nuclear enzyme that relieves torsional stress in chromosomal DNA during various cellular processes. Agents that target Top2, involving etoposide, doxorubicin, and mitoxantrone, are among the most effective anticancer drugs used in the clinic. Mammalian cells possess two genetically distinct Top2 isoforms, both of which are the target of these agents. Top2alpha is essential for cell proliferation and is highly expressed in vigorously growing cells, whereas Top2beta is nonessential for growth and has recently been implicated in treatment-associated secondary malignancies, highlighting the validity of a Top2alpha-specific drug for future cancer treatment; however, no such agent has been hitherto reported. Here we show that NK314, a novel synthetic benzo[c]phenanthridine alkaloid, targets Top2alpha and not Top2beta in vivo. Unlike other Top2 inhibitors, NK314 induces Top2-DNA complexes and double-strand breaks (DSBs) in an alpha isoform-specific manner. Heterozygous disruption of the human TOP2alpha gene confers increased NK314 resistance, whereas TOP2beta homozygous knock-out cells display increased NK314 sensitivity, indicating that the alpha isoform is the cellular target. We further show that the absence of Top2beta does not alleviate NK314 hypersensitivity of cells deficient in non-homologous end-joining, a critical pathway for repairing Top2-mediated DSBs. Our results indicate that NK314 acts as a Top2alpha-specific poison in mammalian cells, with excellent potential as an efficacious and safe chemotherapeutic agent. We also suggest that a series of human knock-out cell lines are useful in assessing DNA damage and repair induced by potential topoisomerase-targeting agents.
Figures




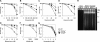
Similar articles
-
NK314, a novel topoisomerase II inhibitor, induces rapid DNA double-strand breaks and exhibits superior antitumor effects against tumors resistant to other topoisomerase II inhibitors.Cancer Lett. 2008 Jan 18;259(1):99-110. doi: 10.1016/j.canlet.2007.10.004. Epub 2007 Nov 12. Cancer Lett. 2008. PMID: 17998154
-
The polyphenolic ellagitannin vescalagin acts as a preferential catalytic inhibitor of the α isoform of human DNA topoisomerase II.Mol Pharmacol. 2012 Jul;82(1):134-41. doi: 10.1124/mol.111.077537. Epub 2012 Apr 23. Mol Pharmacol. 2012. PMID: 22528119
-
NK314 potentiates antitumor activity with adult T-cell leukemia-lymphoma cells by inhibition of dual targets on topoisomerase II{alpha} and DNA-dependent protein kinase.Blood. 2011 Mar 31;117(13):3575-84. doi: 10.1182/blood-2010-02-270439. Epub 2011 Jan 18. Blood. 2011. PMID: 21245486
-
Regulation of topoisomerase II stability and activity by ubiquitination and SUMOylation: clinical implications for cancer chemotherapy.Mol Biol Rep. 2021 Sep;48(9):6589-6601. doi: 10.1007/s11033-021-06665-7. Epub 2021 Sep 2. Mol Biol Rep. 2021. PMID: 34476738 Free PMC article. Review.
-
Topoisomerase IIα, rather than IIβ, is a promising target in development of anti-cancer drugs.Drug Discov Ther. 2012 Oct;6(5):230-7. Drug Discov Ther. 2012. PMID: 23229142 Review.
Cited by
-
Do transcription factories and TOP2B provide a recipe for chromosome translocations in therapy-related leukemia?Cell Cycle. 2012 Sep 1;11(17):3143-4. doi: 10.4161/cc.21477. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22894901 Free PMC article.
-
TOP2A overexpression as a poor prognostic factor in patients with nasopharyngeal carcinoma.Tumour Biol. 2014 Jan;35(1):179-87. doi: 10.1007/s13277-013-1022-6. Epub 2013 Jul 30. Tumour Biol. 2014. PMID: 23897556
-
Model for MLL translocations in therapy-related leukemia involving topoisomerase IIβ-mediated DNA strand breaks and gene proximity.Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):8989-94. doi: 10.1073/pnas.1204406109. Epub 2012 May 21. Proc Natl Acad Sci U S A. 2012. PMID: 22615413 Free PMC article.
-
Synthesis, Bacteriostatic and Anticancer Activity of Novel Phenanthridines Structurally Similar to Benzo[c]phenanthridine Alkaloids.Molecules. 2018 Aug 27;23(9):2155. doi: 10.3390/molecules23092155. Molecules. 2018. PMID: 30150591 Free PMC article.
-
Topoisomerase II and leukemia.Ann N Y Acad Sci. 2014 Mar;1310(1):98-110. doi: 10.1111/nyas.12358. Epub 2014 Feb 3. Ann N Y Acad Sci. 2014. PMID: 24495080 Free PMC article. Review.
References
-
- Wang, J. C. (1996) Annu. Rev. Biochem. 65635 –692 - PubMed
-
- Dong, K. C., and Berger, J. M. (2007) Nature 4501201 –1205 - PubMed
-
- Wang, J. C. (2002) Nat. Rev. Mol. Cell Biol. 3430 –440 - PubMed
-
- Li, T. K., and Liu, L. F. (2001) Annu. Rev. Pharmacol. Toxicol. 4153 –77 - PubMed
-
- Osheroff, N. (1989) Biochemistry 286157 –6160 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

