The Werner syndrome protein binds replication fork and holliday junction DNAs as an oligomer
- PMID: 18596042
- PMCID: PMC2528990
- DOI: 10.1074/jbc.M803370200
The Werner syndrome protein binds replication fork and holliday junction DNAs as an oligomer
Abstract
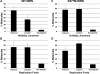
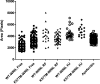
Werner syndrome is an inherited disease displaying a premature aging phenotype. The gene mutated in Werner syndrome encodes both a 3' --> 5' DNA helicase and a 3' --> 5' DNA exonuclease. Both WRN helicase and exonuclease preferentially utilize DNA substrates containing alternate secondary structures. By virtue of its ability to resolve such DNA structures, WRN is postulated to prevent the stalling and collapse of replication forks that encounter damaged DNA. Using electron microscopy, we visualized the binding of full-length WRN to DNA templates containing replication forks and Holliday junctions, intermediates observed during DNA replication and recombination, respectively. We show that both wild-type WRN and a helicase-defective mutant bind with exceptionally high specificity (>1000-fold) to DNA secondary structures at the replication fork and at Holliday junctions. Little or no binding is observed elsewhere on the DNA molecules. Calculations of the molecular weight of full-length WRN revealed that, in solution, WRN exists predominantly as a dimer. However, WRN bound to DNA is larger; the mass is consistent with that of a tetramer.
Figures



References
-
- Singleton, M. R., Dillingham, M. S., and Wigley, D. B. (2007) Annu. Rev. Biochem. 7623 –50 - PubMed
-
- Ozgenc, A., and Loeb, L. A. (2005) Mutat. Res. 577237 –251 - PubMed
-
- Kamath-Loeb, A. S., Fry, M., and Loeb, L. A. (2006) DNA Helicases and Human Disease, pp.435 –460, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

