Autophagosome supports coxsackievirus B3 replication in host cells
- PMID: 18596087
- PMCID: PMC2546883
- DOI: 10.1128/JVI.00641-08
Autophagosome supports coxsackievirus B3 replication in host cells
Abstract
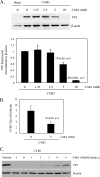
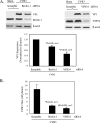
Recent studies suggest a possible takeover of host antimicrobial autophagy machinery by positive-stranded RNA viruses to facilitate their own replication. In the present study, we investigated the role of autophagy in coxsackievirus replication. Coxsackievirus B3 (CVB3), a picornavirus associated with viral myocarditis, causes pronounced intracellular membrane reorganization after infection. We demonstrate that CVB3 infection induces an increased number of double-membrane vesicles, accompanied by an increase of the LC3-II/LC3-I ratio and an accumulation of punctate GFP-LC3-expressing cells, two hallmarks of cellular autophagosome formation. However, protein expression analysis of p62, a marker for autophagy-mediated protein degradation, showed no apparent changes after CVB3 infection. These results suggest that CVB3 infection triggers autophagosome formation without promoting protein degradation by the lysosome. We further examined the role of the autophagosome in CVB3 replication. We demonstrated that inhibition of autophagosome formation by 3-methyladenine or small interfering RNAs targeting the genes critical for autophagosome formation (ATG7, Beclin-1, and VPS34 genes) significantly reduced viral replication. Conversely, induction of autophagy by rapamycin or nutrient deprivation resulted in increased viral replication. Finally, we examined the role of autophagosome-lysosome fusion in viral replication. We showed that blockage of the fusion by gene silencing of the lysosomal protein LAMP2 significantly promoted viral replication. Taken together, our results suggest that the host's autophagy machinery is activated during CVB3 infection to enhance the efficiency of viral replication.
Figures







References
-
- Blommaart, E. F., U. Krause, J. P. Schellens, H. Vreeling-Sindelarova, and A. J. Meijer. 1997. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243240-246. - PubMed
-
- Codogno, P., and A. J. Meijer. 2005. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12(Suppl. 2)1509-1518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

