Comprehensive characterization of extracellular herpes simplex virus type 1 virions
- PMID: 18596102
- PMCID: PMC2519676
- DOI: 10.1128/JVI.00904-08
Comprehensive characterization of extracellular herpes simplex virus type 1 virions
Abstract
The herpes simplex virus type 1 (HSV-1) genome is contained in a capsid wrapped by a complex tegument layer and an external envelope. The poorly defined tegument plays a critical role throughout the viral life cycle, including delivery of capsids to the nucleus, viral gene expression, capsid egress, and acquisition of the viral envelope. Current data suggest tegumentation is a dynamic and sequential process that starts in the nucleus and continues in the cytoplasm. Over two dozen proteins are assumed to be or are known to ultimately be added to virions as tegument, but its precise composition is currently unknown. Moreover, a comprehensive analysis of all proteins found in HSV-1 virions is still lacking. To better understand the implication of the tegument and host proteins incorporated into the virions, highly purified mature extracellular viruses were analyzed by mass spectrometry. The method proved accurate (95%) and sensitive and hinted at 8 different viral capsid proteins, 13 viral glycoproteins, and 23 potential viral teguments. Interestingly, four novel virion components were identified (U(L)7, U(L)23, U(L)50, and U(L)55), and two teguments were confirmed (ICP0 and ICP4). In contrast, U(L)4, U(L)24, the U(L)31/U(L)34 complex, and the viral U(L)15/U(L)28/U(L)33 terminase were undetected, as was most of the viral replication machinery, with the notable exception of U(L)23. Surprisingly, the viral glycoproteins gJ, gK, gN, and U(L)43 were absent. Analyses of virions produced by two unrelated cell lines suggest their protein compositions are largely cell type independent. Finally, but not least, up to 49 distinct host proteins were identified in the virions.
Figures
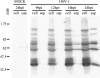
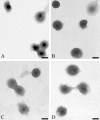
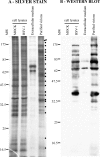




References
-
- Adams, R., C. Cunningham, M. D. Davison, C. A. MacLean, and A. J. Davison. 1998. Characterization of the protein encoded by gene UL49A of herpes simplex virus type 1. J. Gen. Virol. 79813-823. - PubMed
-
- America, A. H., and J. H. Cordewener. 2008. Comparative LC-MS: a landscape of peaks and valleys. Proteomics 8731-749. - PubMed
-
- Anderson, L., and C. L. Hunter. 2006. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 5573-588. - PubMed
-
- Baines, J., and C. Duffy. 2006. Nucleocapsid assembly and envelopment of herpes simplex virus, p. 175-204. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

