Vaccinia virus l1 protein is required for cell entry and membrane fusion
- PMID: 18596103
- PMCID: PMC2519644
- DOI: 10.1128/JVI.00852-08
Vaccinia virus l1 protein is required for cell entry and membrane fusion
Abstract
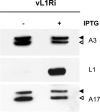
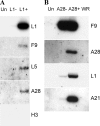
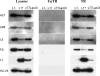
Genetic and biochemical studies have provided evidence for an entry/fusion complex (EFC) comprised of at least eight viral proteins (A16, A21, A28, G3, G9, H2, J5, and L5) that together with an associated protein (F9) participates in entry of vaccinia virus (VACV) into cells. The genes encoding these proteins are conserved in all poxviruses, are expressed late in infection, and are components of the mature virion membrane but are not required for viral morphogenesis. In addition, all but one component has intramolecular disulfides that are formed by the poxvirus cytoplasmic redox system. The L1 protein has each of the characteristics enumerated above except that it has been reported to be essential for virus assembly. To further investigate the role of L1, we constructed a recombinant VACV (vL1Ri) that inducibly expresses L1. In the absence of inducer, L1 synthesis was repressed and vL1Ri was unable to form plaques or produce infectious progeny. Unexpectedly, assembly and morphogenesis appeared normal and the noninfectious virus particles were indistinguishable from wild-type VACV as determined by transmission electron microscopy and analysis of the component polypeptides. Notably, the L1-deficient virions were able to attach to cells but the cores failed to penetrate into the cytoplasm. In addition, cells infected with vL1Ri in the absence of inducer did not form syncytia following brief low-pH treatment even though extracellular virus was produced. Coimmunoprecipitation experiments demonstrated that L1 interacted with the EFC and indirectly with F9, suggesting that L1 is an additional component of the viral entry apparatus.
Figures





References
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 34159-71. - PubMed
-
- Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 139-17. - PubMed
-
- Betakova, T., E. J. Wolffe, and B. Moss. 1999. Membrane topology of the vaccinia virus A17L envelope protein. Virology 261347-356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

