Successful treatment of class V+IV lupus nephritis with multitarget therapy
- PMID: 18596121
- PMCID: PMC2551567
- DOI: 10.1681/ASN.2007121272
Successful treatment of class V+IV lupus nephritis with multitarget therapy
Abstract
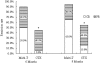
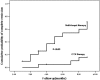
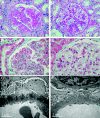
Treatment of class V+IV lupus nephritis remains unsatisfactory despite the progress made in the treatment of diffuse proliferative lupus nephritis. In this prospective study, 40 patients with class V+IV lupus nephritis were randomly assigned to induction therapy with mycophenolate mofetil, tacrolimus, and steroids (multitarget therapy) or intravenous cyclophosphamide (IVCY). Patients were treated for 6 mo unless complete remission was not achieved, in which case treatment was extended to 9 mo. An intention-to-treat analysis revealed a higher rate of complete remission with multitarget therapy at both 6 and 9 mo (50 and 65%, respectively) than with IVCY (5 and 15%, respectively). At 6 mo, eight (40%) patients in each group experienced partial remission, and at 9 mo, six (30%) patients receiving multitarget therapy and eight (40%) patients receiving IVCY experienced partial remission. There were no deaths during this study. Most adverse events were less frequent in the multitarget therapy group. Calcineurin inhibitor nephrotoxicity was not observed, but three patients developed new-onset hypertension with multitarget therapy. In conclusion, multitarget therapy is superior to IVCY for inducing complete remission of class V+IV lupus nephritis and is well tolerated.
Trial registration: ClinicalTrials.gov NCT00298506.
Figures



Comment in
-
Multitarget therapy of lupus nephritis: base hit or home run?J Am Soc Nephrol. 2008 Oct;19(10):1842-4. doi: 10.1681/ASN.2008070779. Epub 2008 Aug 27. J Am Soc Nephrol. 2008. PMID: 18753251 No abstract available.
References
-
- Karim Y, D'Cruz DP: The NIH pulse cyclophosphamide regime: The end of an era? Lupus 13: 1–3, 2004 - PubMed
-
- Li LS, Liu ZH, Zhou H, Hu WX: Mycophenolate mofetil showed inhibitory effect on VACM expression during successful treatment of diffuse proliferative lupus nephritis (SLE-DPGN) with vascular lesions. In: ASN Program and Abstracts, Philadelphia, Williams & Wilkins, 1998, pp 153
-
- Hu W, Liu Z, Chen H, Tang Z, Wang Q, Shen K, Li L: Mycophenolate mofetil vs cyclophosphamide therapy for patients with diffuse proliferative lupus nephritis. Chin Med J (Engl) 115: 705–709, 2002 - PubMed
-
- Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN: Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med 343: 1156–1162, 2000 - PubMed
-
- Najafi CC, Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J: Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int 59: 2156–2163, 2001 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

