Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture
- PMID: 18596122
- PMCID: PMC2551564
- DOI: 10.1681/ASN.2007101087
Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture
Abstract
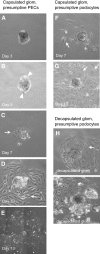
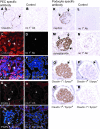
Parietal epithelial cells (PEC) are major constituents of crescents in crescentic glomerulonephritis. The purpose of these studies was to establish an immortalized PEC cell line with similar characteristics to PEC in vivo for use in future mechanistic studies. Glomeruli were isolated from H-2Kb tsA58 transgenic mice (ImmortoMouse) by standard differential sieving, and several candidate PEC cell lines were obtained by subcloning outgrowths of cells from capsulated glomeruli. One clone, designated mouse PEC (mPEC), was extensively characterized. mPEC exhibited a compact cell body with typical epithelial morphology when grown in permissive conditions, but the cell shape changed to polygonal after 14 d in growth-restrictive conditions. mPEC but not podocytes used as a negative control expressed claudin-1, claudin-2, and protein gene product 9.5, which are proteins specific to PEC in vivo, and did not express the podocyte-specific proteins synaptopodin and nephrin. The junctional proteins zonula occludens-1 and beta-catenin stained positively in both mPEC and podocytes, but the staining pattern at cell-cell contacts was intermittent in mPEC and linear in podocytes. Finally, mPEC had thin bundled cortical F-actin filaments and no F-actin projections compared with podocytes, which exhibited thick bundled cortical F-actin filaments and interdigitating F-actin projections at cell-cell contacts. We conclude that immortalized mPEC in culture exhibit specific features of PEC in vivo and that these cells are distinct from podocytes, despite having the same mesenchymal origin. This mPEC line will assist in future mechanistic studies of PEC and enhance our understanding of glomerular injury.
Figures






References
-
- Shimizu M, Kondo S, Urushihara M, Takamatsu M, Kanemoto K, Nagata M, Kagami S: Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol Dial Transplant 21: 2380–2390, 2006 - PubMed
-
- Fujigaki Y, Sun DF, Fujimoto T, Suzuki T, Goto T, Yonemura K, Morioka T, Yaoita E, Hishida A: Mechanisms and kinetics of Bowman's epithelial-myofibroblast transdifferentiation in the formation of glomerular crescents. Nephron 92: 203–212, 2002 - PubMed
-
- Kanemoto K, Usui J, Tomari S, Yokoi H, Mukoyama M, Aten J, Weening JJ, Nagata M: Connective tissue growth factor participates in scar formation of crescentic glomerulonephritis. Lab Invest 83: 1615–1625, 2003 - PubMed
-
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 - PubMed
-
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S: Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

