Synaptic strength modulation after cortical trauma: a role in epileptogenesis
- PMID: 18596152
- PMCID: PMC6670971
- DOI: 10.1523/JNEUROSCI.0643-08.2008
Synaptic strength modulation after cortical trauma: a role in epileptogenesis
Abstract
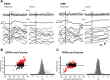
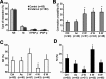
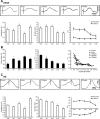
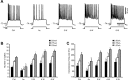
Traumatic brain injuries are often followed by abnormal hyperexcitability, leading to acute seizures and epilepsy. Previous studies documented the rewiring capacity of neocortical neurons in response to various cortical and subcortical lesions. However, little information is available on the functional consequences of these anatomical changes after cortical trauma and the adaptation of synaptic connectivity to a decreased input produced by chronic deafferentation. In this study, we recorded intracellular (IC) activities of cortical neurons simultaneously with extracellular (EC) unit activities and field potentials of neighboring cells in cat cortex, after a large transection of the white matter underneath the suprasylvian gyrus, in acute and chronic conditions (at 2, 4, and 6 weeks) in ketamine-xylazine-anesthetized cats. Using EC spikes to compute the spike-triggered averages of IC membrane potential, we found an increased connection probability and efficacy between cortical neurons weeks after cortical trauma. Inhibitory interactions showed no significant changes in the traumatized cortex compared with control. The increased synaptic efficacy was accompanied by enhanced input resistance and intrinsic excitability of cortical neurons, as well as by increased duration of silent network periods. Our electrophysiological data revealed functional consequences of previously reported anatomical changes in the injured cortex. We suggest that homeostatic synaptic plasticity compensating the decreased activity in the undercut cortex leads to an uncontrollable cortical hyperexcitability and seizure generation.
Figures




Comment in
-
Is modulation of cortical synapses after brain trauma homeostatic? Or, since when is epilepsy normal?Epilepsy Curr. 2009 Mar-Apr;9(2):53-4. doi: 10.1111/j.1535-7511.2008.01290.x. Epilepsy Curr. 2009. PMID: 19421381 Free PMC article. No abstract available.
References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. - PubMed
-
- Avramescu S, Timofeev I. Synaptic synchrony modulation following cortical injury. Soc Neurosci Abstr. 2006;32:235–26.
-
- Bausch SB, He S, Petrova Y, Wang XM, McNamara JO. Plasticity of both excitatory and inhibitory synapses is associated with seizures induced by removal of chronic blockade of activity in cultured hippocampus. J Neurophysiol. 2006;96:2151–2167. - PubMed
-
- Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Cote R, Lebrun L, Pirisi A, Norris JW. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57:1617–1622. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
