A retinal circuit that computes object motion
- PMID: 18596156
- PMCID: PMC6670970
- DOI: 10.1523/JNEUROSCI.4206-07.2008
A retinal circuit that computes object motion
Abstract
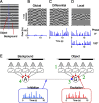
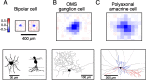
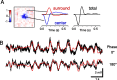
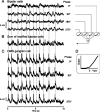
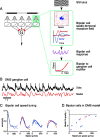
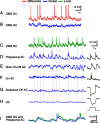
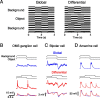
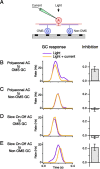
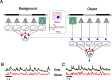
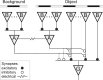
Certain ganglion cells in the retina respond sensitively to differential motion between the receptive field center and surround, as produced by an object moving over the background, but are strongly suppressed by global image motion, as produced by the observer's head or eye movements. We investigated the circuit basis for this object motion sensitive (OMS) response by recording intracellularly from all classes of retinal interneurons while simultaneously recording the spiking output of many ganglion cells. Fast, transient bipolar cells respond linearly to motion in the receptive field center. The synaptic output from their terminals is rectified and then pooled by the OMS ganglion cell. A type of polyaxonal amacrine cell is driven by motion in the surround, again via pooling of rectified inputs, but from a different set of bipolar cell terminals. By direct intracellular current injection, we found that these polyaxonal amacrine cells selectively suppress the synaptic input of OMS ganglion cells. A quantitative model of these circuit elements and their interactions explains how an important visual computation is accomplished by retinal neurons and synapses.
Figures
References
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. - PubMed
-
- Born RT, Tootell RB. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357:497–499. - PubMed
-
- Burkhardt DA, Fahey PK. Contrast enhancement and distributed encoding by bipolar cells in the retina. J Neurophysiol. 1998;80:1070–1081. - PubMed
-
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci. 1998;1:714–719. - PubMed
-
- Costa LF, Velte TJ. Automatic characterization and classification of ganglion cells from the salamander retina. J Comp Neurol. 1999;404:33–51. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
