What grid cells convey about rat location
- PMID: 18596161
- PMCID: PMC6670990
- DOI: 10.1523/JNEUROSCI.5684-07.2008
What grid cells convey about rat location
Abstract
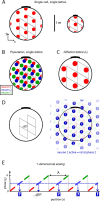
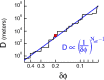
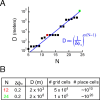
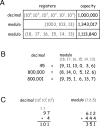
We characterize the relationship between the simultaneously recorded quantities of rodent grid cell firing and the position of the rat. The formalization reveals various properties of grid cell activity when considered as a neural code for representing and updating estimates of the rat's location. We show that, although the spatially periodic response of grid cells appears wasteful, the code is fully combinatorial in capacity. The resulting range for unambiguous position representation is vastly greater than the approximately 1-10 m periods of individual lattices, allowing for unique high-resolution position specification over the behavioral foraging ranges of rats, with excess capacity that could be used for error correction. Next, we show that the merits of the grid cell code for position representation extend well beyond capacity and include arithmetic properties that facilitate position updating. We conclude by considering the numerous implications, for downstream readouts and experimental tests, of the properties of the grid cell code.
Figures


References
-
- Abbott L, Sejnowski T, editors. Neural codes and distributed representations. Cambridge, MA: MIT; 1999.
-
- Alyan S, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113:19–31. - PubMed
-
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. - PubMed
-
- Bajard J-C, Imbert L. A full rns implementation of rsa. IEEE Trans Comput. 2004;53:769–774.
-
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
