Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects
- PMID: 18596168
- PMCID: PMC6670980
- DOI: 10.1523/JNEUROSCI.0542-08.2008
Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects
Abstract
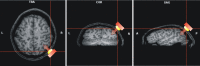
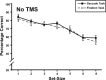
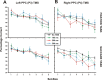
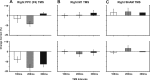
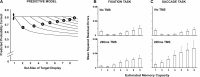
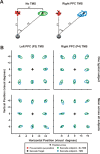
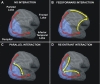
The posterior parietal cortex (PPC) plays a role in spatial updating of goals for eye and arm movements across saccades, but less is known about its role in updating perceptual memory. We reported previously that transsaccadic memory has a capacity for storing the orientations of three to four Gabor patches either within a single fixation (fixation task) or between separate fixations (saccade task). Here, we tested the role of the PPC in transsaccadic memory in eight subjects by simultaneously applying single-pulse transcranial magnetic stimulation (TMS) over the right and left PPC, over several control sites, and comparing these to behavioral controls with no TMS. In TMS trials, we randomly delivered pulses at one of three different time intervals around the time of the saccade, or at an equivalent time in the fixation task. Controls confirmed that subjects could normally retain at least three visual features. TMS over the left PPC and a control site had no significant effect on this performance. However, TMS over the right PPC disrupted memory performance in both tasks. This TMS-induced effect was most disruptive in the saccade task, in particular when stimulation coincided more closely with saccade timing. Here, the capacity to compare presaccadic and postsaccadic features was reduced to one object, as expected if the spatial aspect of memory was disrupted. This finding suggests that right PPC plays a role in the spatial processing involved in transsaccadic memory of visual features. We propose that this process uses saccade-related feedback signals similar to those observed in spatial updating.
Figures

References
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
-
- Beck DM, Muggleton N, Walsh V, Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb Cortex. 2006;16:712–717. - PubMed
-
- Beckers G, Zeki S. The consequences of inactivating areas V1 and V5 on visual motion perception. Brain. 1995;118:49–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
