Toxicity of anthrax toxin is influenced by receptor expression
- PMID: 18596206
- PMCID: PMC2546661
- DOI: 10.1128/CVI.00103-08
Toxicity of anthrax toxin is influenced by receptor expression
Abstract
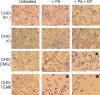
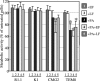
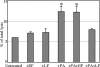
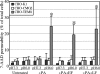
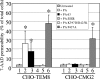
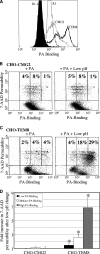
Anthrax toxin protective antigen (PA) binds to its cellular receptor, and seven subunits self-associate to form a heptameric ring that mediates the cytoplasmic entry of lethal factor or edema factor. The influence of receptor type on susceptibility to anthrax toxin components was examined using Chinese hamster ovary (CHO) cells expressing the human form of one of two PA receptors: TEM8 or CMG2. Unexpectedly, PA alone, previously believed to only mediate entry of lethal factor or edema factor, was found to be toxic to CHO-TEM8 cells; cells treated with PA alone displayed reduced cell growth and decreased metabolic activity. PA-treated cells swelled and became permeable to membrane-excluded dye, suggesting that PA formed cell surface pores on CHO-TEM8 cells. While CHO-CMG2 cells were not killed by wild-type PA, they were susceptible to the PA variant, F427A. Receptor expression also conferred differences in susceptibility to edema factor.
Figures

References
-
- Beauregard, K. E., R. J. Collier, and J. A. Swanson. 2000. Proteolytic activation of receptor-bound anthrax protective antigen on macrophages promotes its internalization. Cell. Microbiol. 2:251-258. - PubMed
-
- Bell, S. E., A. Mavila, R. Salazar, K. J. Bayless, S. Kanagala, S. A. Maxwell, and G. E. Davis. 2001. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell Sci. 114:2755-2773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

