Jonathan E Rhoads lecture: Of mice and men... and a few hundred rats
- PMID: 18596320
- PMCID: PMC2596714
- DOI: 10.1177/0148607108319795
Jonathan E Rhoads lecture: Of mice and men... and a few hundred rats
Figures















Similar articles
-
1996 Jonathan E. Rhoads Lecture. Mechanism, mechanism, mechanism.JPEN J Parenter Enteral Nutr. 1996 Sep-Oct;20(5):319-24. doi: 10.1177/0148607196020005319. JPEN J Parenter Enteral Nutr. 1996. PMID: 8887899 No abstract available.
-
Jonathan E. Rhoads Lecture. Medicine, nutrition, and patient care: a panoramic view.JPEN J Parenter Enteral Nutr. 1994 Sep-Oct;18(5):387-95. doi: 10.1177/0148607194018005387. JPEN J Parenter Enteral Nutr. 1994. PMID: 7815667 Review. No abstract available.
-
First annual Jonathan E. Rhoads lecture: Dedicated to Jonathan E. Rhoads for 45 years the purveyor of opportunities for students young and old--in grateful appreciation.JPEN J Parenter Enteral Nutr. 1978 May;2(2):75-6. doi: 10.1177/014860717800200201. JPEN J Parenter Enteral Nutr. 1978. PMID: 121755 No abstract available.
-
Clinical aspects of essential fatty acid metabolism: Jonathan Rhoads Lecture.JPEN J Parenter Enteral Nutr. 2003 May-Jun;27(3):168-75. doi: 10.1177/0148607103027003168. JPEN J Parenter Enteral Nutr. 2003. PMID: 12757109 Review.
-
Jonathan E. Rhoads lecture. Intravenous hyperalimentation and cancer. A historical perspective.JPEN J Parenter Enteral Nutr. 1986 Jul-Aug;10(4):337-42. doi: 10.1177/0148607186010004337. JPEN J Parenter Enteral Nutr. 1986. PMID: 3091858 Review. No abstract available.
Cited by
-
The change in the amount of immunoglobulins as a response to stress experienced by soldiers on a peacekeeping mission.Int Arch Occup Environ Health. 2014 Aug;87(6):615-22. doi: 10.1007/s00420-013-0899-0. Epub 2013 Aug 13. Int Arch Occup Environ Health. 2014. PMID: 23943194
-
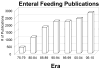
Nutrition and gut immunity.Surg Clin North Am. 2011 Aug;91(4):755-70, vii. doi: 10.1016/j.suc.2011.04.007. Epub 2011 Jun 8. Surg Clin North Am. 2011. PMID: 21787966 Free PMC article. Review.
-
The enteric nervous system neuropeptide, bombesin, reverses innate immune impairments during parenteral nutrition.Ann Surg. 2014 Sep;260(3):432-43; discussion 443-4. doi: 10.1097/SLA.0000000000000871. Ann Surg. 2014. PMID: 25115419 Free PMC article.
References
-
- Rombeau JL, Muldoon D, Jonathan E, Rhoads MD. Quaker sense and sensibility in the world of surgery. Philadelphia: Hanley & Belfus; 1997.
-
- Kudsk KA. Dear Miss Milk Toast (1998 Presidential Address - ASPEN) J Parenter Enteral Nutr. 1998;22(4):191–198. - PubMed
-
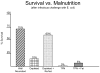
- Peterson SR, Kudsk KA, Carpenter G, Sheldon GF. Malnutrition and Immunocompetence: Increased mortality following an infectious challenge during hyperalimentation. J Trauma. 1981;21:528–533. - PubMed
-
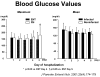
- Kudsk KA, Carpenter BS, Peterson S, Sheldon GF. Effect of Enteral and Parenteral Feeding in Malnourished Rats with E. coli-Hemoglobin Adjuvant Peritonitis. J Surg Res. 1981;31:105–110. - PubMed
-
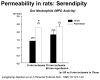
- Kudsk KA, Stone JM, Carpenter BA, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983;23:605–609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

