Fluorescence interferometry: principles and applications in biology
- PMID: 18596334
- PMCID: PMC10902801
- DOI: 10.1196/annals.1430.038
Fluorescence interferometry: principles and applications in biology
Abstract
The use of fluorescence radiation is of fundamental importance for tackling measurement problems in the life sciences, with recent demonstrations of probing biological systems at the nanoscale. Usually, fluorescent light-based tools and techniques use the intensity of light waves, which is easily measured by detectors. However, the phase of a fluorescence wave contains subtle, but no less important, information about the wave; yet, it has been largely unexplored. Here, we introduce the concept of fluorescence interferometry to allow the measurement of phase information of fluorescent light waves. In principle, fluorescence interferometry can be considered a unique form of optical low-coherence interferometry that uses fluorophores as a light source of low temporal coherence. Fluorescence interferometry opens up new avenues for developing new fluorescent light-based imaging, sensing, ranging, and profiling methods that to some extent resemble interferometric techniques based on white light sources. We propose two experimental realizations of fluorescence interferometry that detect the interference pattern cast by the fluorescence fields. This article discusses their measurement capabilities and limitations and compares them with those offered by optical low-coherence interferometric schemes. We also describe applications of fluorescence interferometry to imaging, ranging, and profiling tasks and present experimental evidences of wide-field cross-sectional imaging with high resolution and large range of depth, as well as quantitative profiling with nanometer-level precision. Finally, we point out future research directions in fluorescence interferometry, such as fluorescence tomography of whole organisms and the extension to molecular interferometry by means of quantum dots and bioluminescence.
Conflict of interest statement
Conflict of Interest
The authors declare no conflicts of interest.
Figures

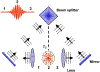
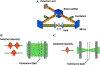


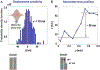
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

