Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus
- PMID: 18596544
- PMCID: PMC4879614
- DOI: 10.1097/NEN.0b013e318181b8ae
Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus
Abstract
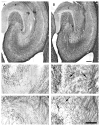
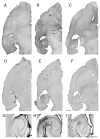
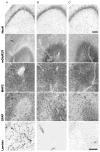
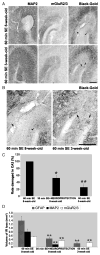
In limbic seizures, neuronal excitation is conveyed from the entorhinal cortex directly to CA1 and subicular regions. This phenomenon is associated with a reduced ability of CA3 to respond to entorhinal cortex inputs. Here, we describe a lesion that destroys the perforant path in CA3 after status epilepticus (SE) induced by pilocarpine injection in 8-week-old rats. Using magnetic resonance imaging, immunohistochemical, and ultrastructural analyses, we determined that this lesion develops after 30 minutes of SE and is characterized by microhemorrhages and ischemia. After a longer period of SE, the lesion invariably involves the upper blade of the dentate gyrus. Adult rats treated with subcutaneous diazepam (20 mg kg for 3 days) did not develop the dentate gyrus lesion and had less frequent spontaneous recurrent seizures (p < 0.01). Young (3-week-old) rats rarely (20%) developed the CA3 lesion, and their spontaneous seizures were delayed (p < 0.01). To investigate the role of the damaged CA3 in seizure activity, we reinduced SE in adult and young epileptic rats. Using FosB/DeltaFosB markers, we found induction of FosB/DeltaFosB immunopositivity in CA3 neurons of young but not in adult rats. These experiments indicate that SE can produce a focal lesion in the perforant path that may affect the roles of the hippocampus in epileptic rats.
Figures





References
-
- Avoli M, D’Antuono M, Louvel J, et al. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system. Prog Neurobiol. 2002;68:167–207. - PubMed
-
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–81. - PubMed
-
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: Rewiring the brain. Prog Neurobiol. 2004;73:1–60. - PubMed
-
- Willoughby JO, Mackenzie L, Medvedev A, et al. Fos induction following systemic kainic acid: Early expression in hippocampus and later widespread expression correlated with seizure. Neuroscience. 1977;77:379–92. - PubMed
-
- Hsieh PF, Watanabe Y. Time course of c-FOS expression in status epilepticus induced by amygdaloid stimulation. Neuroreport. 2000;11:571–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

