Update on recent molecular and genetic advances in frontotemporal lobar degeneration
- PMID: 18596549
- PMCID: PMC2761710
- DOI: 10.1097/NEN.0b013e31817d751c
Update on recent molecular and genetic advances in frontotemporal lobar degeneration
Abstract
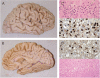
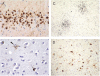
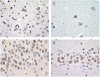
Great strides have been made in the last 2 years in the field of frontotemporal lobar degeneration (FTLD), particularly with respect to the genetics and molecular biology of FTLD with ubiquitinated inclusions. It is now clear that most cases of familial FTLD with ubiquitinated inclusions have mutations in the progranulin gene, located on chromosome 17. It is also clear that most ubiquitinated inclusions in FTLD with ubiquitinated inclusions are composed primarily of TAR DNA-binding protein-43. Thus, FTLDs can be separated into 2 major groups (i.e. tauopathies and ubiquitinopathies), and most of the ubiquitinopathies can now be defined as TAR DNA-binding protein-43 proteinopathies. Many of the familial FTLDs are linked to chromosome 17, including both the familial tauopathies and the familial TAR DNA-binding protein-43 proteinopathies with progranulin mutations. This review highlights the neuropathologic features and the most important discoveries of the last 2 years and places these findings into the historical context of FTLD.
Figures







Similar articles
-
The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments.Neurogenetics. 2007 Nov;8(4):237-48. doi: 10.1007/s10048-007-0102-4. Epub 2007 Sep 6. Neurogenetics. 2007. PMID: 17805587 Review.
-
The genetics of frontotemporal lobar degeneration.Curr Neurol Neurosci Rep. 2007 Sep;7(5):434-42. doi: 10.1007/s11910-007-0067-6. Curr Neurol Neurosci Rep. 2007. PMID: 17764635 Review.
-
Pathology and genetics of frontotemporal lobar degeneration: an update.Clin Neuropathol. 2007 Jul-Aug;26(4):143-56. doi: 10.5414/npp26143. Clin Neuropathol. 2007. PMID: 17702495 Review.
-
Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy.J Neural Transm (Vienna). 2008 Dec;115(12):1661-71. doi: 10.1007/s00702-008-0137-1. Epub 2008 Oct 31. J Neural Transm (Vienna). 2008. PMID: 18974920 Free PMC article.
-
The complex aetiology of frontotemporal lobar degeneration.Exp Neurol. 2007 Jul;206(1):1-10. doi: 10.1016/j.expneurol.2007.03.017. Epub 2007 Mar 24. Exp Neurol. 2007. PMID: 17509568 Review.
Cited by
-
Twin pairs discordant for neuropathologically confirmed Lewy body dementia.J Neurol Neurosurg Psychiatry. 2009 May;80(5):562-5. doi: 10.1136/jnnp.2008.151654. J Neurol Neurosurg Psychiatry. 2009. PMID: 19372291 Free PMC article.
-
Frontotemporal dementias: Recent advances and current controversies.Ann Indian Acad Neurol. 2010 Dec;13(Suppl 2):S74-80. doi: 10.4103/0972-2327.74249. Ann Indian Acad Neurol. 2010. PMID: 21369422 Free PMC article.
-
Clinical and pathological continuum of multisystem TDP-43 proteinopathies.Arch Neurol. 2009 Feb;66(2):180-9. doi: 10.1001/archneurol.2008.558. Arch Neurol. 2009. PMID: 19204154 Free PMC article.
-
Ante-mortem plasma phosphorylated tau (181) predicts Alzheimer's disease neuropathology and regional tau at autopsy.Brain. 2022 Oct 21;145(10):3546-3557. doi: 10.1093/brain/awac175. Brain. 2022. PMID: 35554506 Free PMC article.
-
Decreased myelin proteins in brain donors exposed to football-related repetitive head impacts.Brain Commun. 2023 Mar 6;5(2):fcad019. doi: 10.1093/braincomms/fcad019. eCollection 2023. Brain Commun. 2023. PMID: 36895961 Free PMC article.
References
-
- Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr. 1892;17:165–67.
-
- Alzheimer A. Über eigenartige Krankheitsfȧlle des späteren Alters. Z Gesamte Neurol Psychiat. 1911;4:356–85.
-
- Gans A. Betrachtungen über art und Ausbreitung des krankhaften Prozesses in einem Fall van Pickscher Atrophie des Stirnhins. Zeitschr f d ges Neurol u Psychiatr (Berl) 1922;80:10–28.
-
- Brun A, Gustafson L, Risberg J, et al. Frontal lobe dementia of non-Alzheimer type. Arch Gerontol Geriatr. 1987;6:193–208. - PubMed
-
- Knopman DS, Mastri AR, Frey WH, 2nd, et al. Dementia lacking distinctive histologic features: A common non-Alzheimer degenerative dementia. Neurology. 1990;40:251–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

