Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity
- PMID: 18596648
- PMCID: PMC6245398
- DOI: 10.3390/molecules13061219
Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity
Abstract
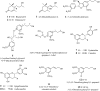
Dragon's blood (Sangre de drago), a viscous red sap derived from Croton lechleri Muell-Arg (Euphorbiaceae), is extensively used by indigenous cultures of the Amazonian basin for its wound healing properties. The aim of this study was to identify the minor secondary metabolites and test the antioxidant activity of this sustance. A bioguided fractionation of the n-hexane, chloroform, n-butanol, and aqueous extracts led to the isolation of 15 compounds: three megastigmanes, four flavan-3-ols, three phenylpropanoids, three lignans, a clerodane, and the alkaloid taspine. In addition to these known molecules, six compounds were isolated and identified for the first time in the latex: blumenol B, blumenol C, 4,5-dihydroblumenol A, erythro-guaiacyl-glyceryl-beta-O-4'- dihydroconiferyl ether, 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol and floribundic acid glucoside. Combinations of spectroscopic methods ((1)H-, (13)C- NMR and 2D-NMR experiments), ESI-MS, and literature comparisons were used for compound identification. In vitro antioxidant activities were assessed by DPPH, total antioxidant capacity and lipid peroxidation assays. Flavan-3-ols derivatives (as major phenolic compounds in the latex) exhibited the highest antioxidant activity.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous