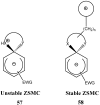
Stable spirocyclic Meisenheimer complexes
- PMID: 18596655
- PMCID: PMC6245453
- DOI: 10.3390/molecules13061282
Stable spirocyclic Meisenheimer complexes
Abstract
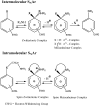
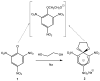
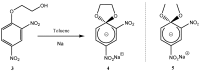
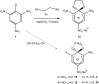
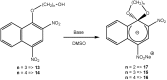
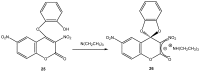
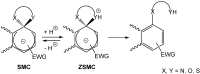
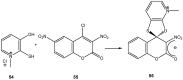
Meisenheimer complexes are important intermediates in Nucleophilic Aromatic Substitution Reactions (S(N)Ar). They are formed by the addition of electron rich species to polynitro aromatic compounds or aromatic compounds with strong electron withdrawing groups. It is possible to distinguish two types of Meisenheimer or sigma-complexes, the sigma(H)-complex or sigma(X)-complex (also named ipso), depending on the aromatic ring position attacked by the nucleophile (a non-substituted or substituted one, respectively). Special examples of sigma(X)- or ipso-complexes are formed through intermediate spiro adducts, via intramolecular SNAr. Some of these spirocyclic Meisenheimer complexes, a type of sigma(X)-complex, are exceptionally stable in solution and/or as solids. They can be isolated and characterized using X-ray, and various spectroscopic techniques such as NMR, UV-Vis, IR, and fluorescence. A few of these stable spirocyclic Meisenheimer complexes are zwitterionic and exhibit interesting photophysical and redox properties. We will review recent advances, synthesis and potential applications of these stable spirocyclic Meisenheimer complexes.
Figures
References
-
- Bunnett J. F., Zahler R. E. Aromatic Nucleophilic Substitution Reactions. Chem. Rev. 1951;49:273–412. doi: 10.1021/cr60153a002. - DOI
-
- Jackson C. J., Gazzolo F. H. Certain coloured substances derived from nitro-compounds. Amer. Chem. J. 1900;23:376.
-
- Meisenheimer J. Reactions of aromatic nitro structures. Justus Liebigs Ann. Chem. 1902:205. doi: 10.1002/jlac.19023230205. - DOI
-
- Foster R., Fyfe C. A. Meisenheimer and related compounds. Rev. Pure Appl. Chem. 1966;16:61.
-
- Buncel E., Norris A. R., Russell K. E. The interaction of aromatic nitro compounds with bases. Quart. Rev. Chem. Soc. 1968;22:123. doi: 10.1039/qr9682200123. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials