Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal
- PMID: 18596687
- PMCID: PMC3660858
- DOI: 10.1038/npp.2008.102
Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal
Abstract
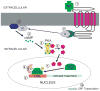
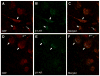
We previously showed that betaxolol, a selective beta(1)-adrenergic receptor antagonist, administered during early phases of cocaine abstinence, ameliorated withdrawal-induced anxiety and blocked increases in amygdalar beta(1)-adrenergic receptor expression in rats. Here, we report the efficacy of betaxolol in reducing increases in gene expression of amygdalar corticotropin-releasing factor (CRF), a peptide known to be involved in mediating 'anxiety-like' behaviors during initial phases of cocaine abstinence. We also demonstrate attenuation of an amygdalar beta(1)-adrenergic receptor-mediated cell-signaling pathway following this treatment. Male rats were administered betaxolol at 24 and 44 h following chronic cocaine administration. Animals were euthanized at the 48-h time point and the amygdala was microdissected and processed for quantitative reverse transcriptase-polymerase chain reaction and/or western blot analysis. Results showed that betaxolol treatment during early cocaine withdrawal attenuated increases in amygdalar CRF gene expression and cyclic adenosine monophosphate-dependent protein kinase regulatory and catalytic subunit (nuclear fraction) protein expression. Our data also reveal that beta(1)-adrenergic receptors are on amygdalar neurons, which are immunoreactive for CRF. The present findings suggest that the efficacy of betaxolol treatment on cocaine withdrawal-induced anxiety may be related, in part, to its effect on amygdalar beta(1)-adrenergic receptor, modulation of its downstream cell-signaling elements and CRF gene expression.
Conflict of interest statement
The authors would like to state that no prior, current or pending conflict of interest exists for any of the authors (Carla A. Rudoy, Arith-Ruth S. Reyes and Elisabeth J. Van Bockstaele) pertaining to the research contained in the present manuscript submission. Furthermore, the authors declare that except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Figures






Similar articles
-
Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats.Prog Neuropsychopharmacol Biol Psychiatry. 2007 Jun 30;31(5):1119-29. doi: 10.1016/j.pnpbp.2007.04.005. Epub 2007 Apr 19. Prog Neuropsychopharmacol Biol Psychiatry. 2007. PMID: 17513029 Free PMC article.
-
Regulation of CRF mRNA in the Rat Extended Amygdala Following Chronic Cocaine: Sex Differences and Effect of Delta Opioid Receptor Agonism.Int J Neuropsychopharmacol. 2020 Feb 1;23(2):117-124. doi: 10.1093/ijnp/pyz067. Int J Neuropsychopharmacol. 2020. PMID: 31867624 Free PMC article.
-
Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA.Eur J Neurosci. 2006 Sep;24(6):1733-43. doi: 10.1111/j.1460-9568.2006.05049.x. Eur J Neurosci. 2006. PMID: 17004937
-
Localization of the delta opioid receptor and corticotropin-releasing factor in the amygdalar complex: role in anxiety.Brain Struct Funct. 2017 Mar;222(2):1007-1026. doi: 10.1007/s00429-016-1261-6. Epub 2016 Jul 4. Brain Struct Funct. 2017. PMID: 27376372 Free PMC article.
-
Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence.Brain Struct Funct. 2008 Sep;213(1-2):43-61. doi: 10.1007/s00429-008-0191-3. Epub 2008 Jul 24. Brain Struct Funct. 2008. PMID: 18651175 Free PMC article. Review.
Cited by
-
Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward.Front Psychiatry. 2013 May 29;4:42. doi: 10.3389/fpsyt.2013.00042. eCollection 2013. Front Psychiatry. 2013. PMID: 23755023 Free PMC article.
-
Paternal methamphetamine exposure induces higher sensitivity to methamphetamine in male offspring through driving ADRB1 on CaMKII-positive neurons in mPFC.Transl Psychiatry. 2023 Oct 19;13(1):324. doi: 10.1038/s41398-023-02624-x. Transl Psychiatry. 2023. PMID: 37857642 Free PMC article.
-
Chronic ethanol alters adrenergic receptor gene expression and produces cognitive deficits in male mice.Neurobiol Stress. 2023 Apr 27;24:100542. doi: 10.1016/j.ynstr.2023.100542. eCollection 2023 May. Neurobiol Stress. 2023. PMID: 37197395 Free PMC article.
-
Larval zebrafish model for FDA-approved drug repositioning for tobacco dependence treatment.PLoS One. 2014 Mar 21;9(3):e90467. doi: 10.1371/journal.pone.0090467. eCollection 2014. PLoS One. 2014. PMID: 24658307 Free PMC article.
-
Chronic REM Sleep Restriction in Juvenile Male Rats Induces Anxiety-Like Behavior and Alters Monoamine Systems in the Amygdala and Hippocampus.Mol Neurobiol. 2018 Apr;55(4):2884-2896. doi: 10.1007/s12035-017-0541-3. Epub 2017 Apr 28. Mol Neurobiol. 2018. PMID: 28455701
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York: 2002. Chapter 15: Cell Communication.
-
- Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006;1106:63–71. - PubMed
-
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
-
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl) 2005;180:781–788. - PubMed
-
- Bourgeais L, Gauriau C, Bernard JF. Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci. 2001;14:229–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

