Viral membrane fusion
- PMID: 18596815
- PMCID: PMC2517140
- DOI: 10.1038/nsmb.1456
Viral membrane fusion
Abstract
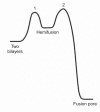
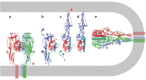
Infection by viruses having lipid-bilayer envelopes proceeds through fusion of the viral membrane with a membrane of the target cell. Viral 'fusion proteins' facilitate this process. They vary greatly in structure, but all seem to have a common mechanism of action, in which a ligand-triggered, large-scale conformational change in the fusion protein is coupled to apposition and merger of the two bilayers. We describe three examples--the influenza virus hemagglutinin, the flavivirus E protein and the vesicular stomatitis virus G protein--in some detail, to illustrate the ways in which different structures have evolved to implement this common mechanism. Fusion inhibitors can be effective antiviral agents.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

