Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model
- PMID: 18596829
- PMCID: PMC2766853
- DOI: 10.1038/gt.2008.101
Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model
Abstract
Cell-based therapy for cancer is a promising new field. Among cell types that can be used for this purpose, mesenchymal stem cells (MSCs) appear to hold great advantage for reasons including easier propagation in culture, possible genetic modification to express therapeutic proteins and preferential homing to sites of cancer growth upon in vivo transfer. The present study evaluated the potential of genetically modified MSC, constitutively expressing interferon (IFN)-beta, in an immunocompetent mouse model of prostate cancer lung metastasis. A recombinant adeno-associated virus (rAAV) encoding mouse IFN-beta was constructed and initially tested in vitro for high-level expression and bioactivity of the transgenic protein. MSCs were transduced by the rAAV-IFN-beta or green fluorescent protein ex vivo and used as cellular vehicles to target lung metastasis of TRAMP-C2 prostate cancer cells in a therapy model. Cohorts of mice were killed on days 30 and 75 to determine the effect of therapy by measurement of tumor volume, histology, immunohistochemistry, enzyme-linked immunosorbent assay and flow cytometry. Results indicated a significant reduction in tumor volume in lungs following IFN-beta-expressing MSC therapy. Immunohistochemistry of the lung demonstrated increased tumor cell apoptosis and decreased tumor cell proliferation and blood vessel counts. A significant increase in the natural kill cell activity was observed following IFN-beta therapy correlating the antitumor effect. Systemic level of IFN-beta was not significantly elevated from this targeted cell therapy. These data demonstrate the potential of MSC-based IFN-beta therapy for prostate cancer lung metastasis.
Figures

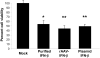





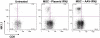
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Miller DC, Spencer BA, Ritchey J, et al. Treatment choice and quality of care for men with localized prostate cancer. Med Care. 2007;45:401–409. - PubMed
-
- Webster WS, Small EJ, Rini BI, Kwon ED. Prostate cancer immunology: biology, therapeutics, and challenges. J Clin Oncol. 2005;23:8262–8269. - PubMed
-
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. - PubMed
-
- Dong Z, Greene G, Pettaway C, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-β. Cancer Research. 1999;59:872–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

