Molecular pathogenesis of pulmonary arterial hypertension
- PMID: 18596905
- PMCID: PMC2439479
- DOI: 10.1172/JCI33452
Molecular pathogenesis of pulmonary arterial hypertension
Abstract
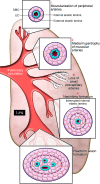
Recent investigations have suggested that it might be possible to reverse the pathology of pulmonary arterial hypertension (PAH), a disorder that can be rapidly progressive and fatal despite current treatments including i.v. prostacyclin. This review will address the cellular and molecular processes implicated in clinical, genetic, and experimental studies as underlying the pulmonary vascular abnormalities associated with PAH. Emerging treatments are aimed at inducing apoptosis of abnormal vascular cells that obstruct blood flow and at promoting regeneration of "lost" distal vasculature.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

