Autoimmune pancreatocholangitis, non-autoimmune pancreatitis and primary sclerosing cholangitis: a comparative morphological and immunological analysis
- PMID: 18596913
- PMCID: PMC2440515
- DOI: 10.1371/journal.pone.0002539
Autoimmune pancreatocholangitis, non-autoimmune pancreatitis and primary sclerosing cholangitis: a comparative morphological and immunological analysis
Abstract
Background: Autoimmune pancreatocholangitis (AIPC) is an emerging, not completely characterized disease. Aim of this study was the comprehensive evaluation of a series of AIPC patients, who were diagnosed and treated in a European institution between January 2003 and July 2006.
Methodology/principal findings: Thirty-three patients with histologically confirmed AIPC were analyzed and compared to 20 patients with non-autoimmune chronic pancreatitis (CP) and 14 patients with primary sclerosing cholangitis (PSC). Clinical features and conventional histopathology were taken into account. Immunohistochemistry and real-time quantitative PCR were used for the characterization of the inflammatory infiltrate and the stromal reaction. AIPC was localized in the pancreatic head in 94% of the patients. Intra- and/or extrapancreatic biliary tract involvement was present in 64% of the cases. The number of infiltrating T-lymphocytes, macrophages and total plasma cells was significantly higher in AIPC than in CP (3-, 4- and 8-fold increase, respectively). The absolute number of IgG4-positive plasma cells was higher in AIPC than in CP and PSC (7-fold and 35-fold increase, respectively), but significance was only reached in comparison with PSC. CXCR5- and CXCL13-positive cells were almost exclusively detected in AIPC.
Conclusions/significance: AIPC is mainly a disease of the pancreatic head with possible extension into the periphery of the gland and/or into the biliary tract/gallbladder. The morphology of AIPC, as well as the immune- and stromal reaction is characteristic and comparable between cases with and without biliary tract involvement. Immunological markers (IgG4, CXCR5, CXCL13) can be of diagnostic relevance in specific settings.
Conflict of interest statement
Figures
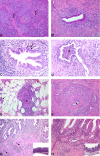

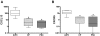

References
-
- Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas–an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. - PubMed
-
- Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. - PubMed
-
- Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. - PubMed
-
- van Buuren HR, Vleggaar FP, Willemien Erkelens G, Zondervan PE, Lesterhuis W, et al. Autoimmune pancreatocholangitis: a series of ten patients. Scand J Gastroenterol. 2006;(Suppl):70–78. - PubMed
-
- Zen Y, Fujii T, Harada K, Kawano M, Yamada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

