KLF6-SV1 overexpression accelerates human and mouse prostate cancer progression and metastasis
- PMID: 18596922
- PMCID: PMC2441856
- DOI: 10.1172/JCI34780
KLF6-SV1 overexpression accelerates human and mouse prostate cancer progression and metastasis
Abstract
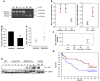
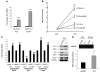
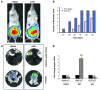
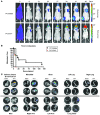
Metastatic prostate cancer (PCa) is one of the leading causes of death from cancer in men. The molecular mechanisms underlying the transition from localized tumor to hormone-refractory metastatic PCa remain largely unknown, and their identification is key for predicting prognosis and targeted therapy. Here we demonstrated that increased expression of a splice variant of the Kruppel-like factor 6 (KLF6) tumor suppressor gene, known as KLF6-SV1, in tumors from men after prostatectomy predicted markedly poorer survival and disease recurrence profiles. Analysis of tumor samples revealed that KLF6-SV1 levels were specifically upregulated in hormone-refractory metastatic PCa. In 2 complementary mouse models of metastatic PCa, KLF6-SV1-overexpressing PCa cells were shown by in vivo and ex vivo bioluminescent imaging to metastasize more rapidly and to disseminate to lymph nodes, bone, and brain more often. Interestingly, while KLF6-SV1 overexpression increased metastasis, it did not affect localized tumor growth. KLF6-SV1 inhibition using RNAi induced spontaneous apoptosis in cultured PCa cell lines and suppressed tumor growth in mice. Together, these findings demonstrate that KLF6-SV1 expression levels in PCa tumors at the time of diagnosis can predict the metastatic behavior of the tumor; thus, KLF-SV1 may represent a novel therapeutic target.
Figures


Similar articles
-
Targeted reduction of KLF6-SV1 restores chemotherapy sensitivity in resistant lung adenocarcinoma.Lung Cancer. 2009 Dec;66(3):292-7. doi: 10.1016/j.lungcan.2009.02.014. Epub 2009 Mar 28. Lung Cancer. 2009. PMID: 19328586 Free PMC article.
-
A small interfering RNA targeting the KLF6 splice variant, KLF6-SV1, as gene therapy for gastric cancer.Gastric Cancer. 2011 Oct;14(4):339-52. doi: 10.1007/s10120-011-0049-x. Epub 2011 May 3. Gastric Cancer. 2011. PMID: 21538018
-
Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread.Cancer Res. 2005 Jul 1;65(13):5761-8. doi: 10.1158/0008-5472.CAN-05-0217. Cancer Res. 2005. PMID: 15994951
-
The role of KLF6 and its splice variants in cancer therapy.Drug Resist Updat. 2009 Feb-Apr;12(1-2):1-7. doi: 10.1016/j.drup.2008.11.001. Epub 2008 Dec 18. Drug Resist Updat. 2009. PMID: 19097929 Review.
-
Biology of Krüppel-like factor 6 transcriptional regulator in cell life and death.IUBMB Life. 2010 Dec;62(12):896-905. doi: 10.1002/iub.396. IUBMB Life. 2010. PMID: 21154818 Review.
Cited by
-
KLF6-SV1 drives breast cancer metastasis and is associated with poor survival.Sci Transl Med. 2013 Jan 23;5(169):169ra12. doi: 10.1126/scitranslmed.3004688. Sci Transl Med. 2013. PMID: 23345610 Free PMC article.
-
Role of Krüppel-like factors in cancer stem cells.J Physiol Biochem. 2015 Mar;71(1):155-64. doi: 10.1007/s13105-015-0381-4. Epub 2015 Jan 24. J Physiol Biochem. 2015. PMID: 25616500 Review.
-
Krüppel-like factors in cancer progression: three fingers on the steering wheel.Oncotarget. 2014 Jan 15;5(1):29-48. doi: 10.18632/oncotarget.1456. Oncotarget. 2014. PMID: 24429391 Free PMC article. Review.
-
Inhibition of proliferation of rat lens epithelial cell by overexpression of KLF6.Mol Vis. 2011 Apr 27;17:1080-4. Mol Vis. 2011. PMID: 21552502 Free PMC article.
-
KLF6 loss of function in human prostate cancer progression is implicated in resistance to androgen deprivation.Am J Pathol. 2012 Sep;181(3):1007-16. doi: 10.1016/j.ajpath.2012.06.008. Epub 2012 Jul 20. Am J Pathol. 2012. PMID: 22819534 Free PMC article.
References
-
- Jemal A., et al. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

