The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone
- PMID: 18596923
- PMCID: PMC2441857
- DOI: 10.1172/JCI35164
The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone
Abstract
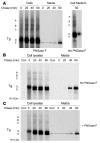
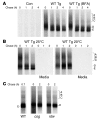
Thyroid hormonogenesis requires secretion of thyroglobulin, a protein comprising Cys-rich regions I, II, and III (referred to collectively as region I-II-III) followed by a cholinesterase-like (ChEL) domain. Secretion of mature thyroglobulin requires extensive folding and glycosylation in the ER. Multiple reports have linked mutations in the ChEL domain to congenital hypothyroidism in humans and rodents; these mutations block thyroglobulin from exiting the ER and induce ER stress. We report that, in a cell-based system, mutations in the ChEL domain impaired folding of thyroglobulin region I-II-III. Truncated thyroglobulin devoid of the ChEL domain was incompetent for cellular export; however, a recombinant ChEL protein ("secretory ChEL") was secreted efficiently. Coexpression of secretory ChEL with truncated thyroglobulin increased intracellular folding, promoted oxidative maturation, and facilitated secretion of region I-II-III, indicating that the ChEL domain may function as an intramolecular chaperone. Additionally, we found that the I-II-III peptide was cosecreted and physically associated with secretory ChEL. A functional ChEL domain engineered to be retained intracellularly triggered oxidative maturation of I-II-III but coretained I-II-III, indicating that the ChEL domain may also function as a molecular escort. These insights into the role of the ChEL domain may represent potential therapeutic targets in the treatment of congenital hypothyroidism.
Figures

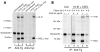

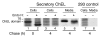





Similar articles
-
Repeat motif-containing regions within thyroglobulin.J Biol Chem. 2011 Jul 29;286(30):26327-33. doi: 10.1074/jbc.M111.242099. Epub 2011 Jun 2. J Biol Chem. 2011. PMID: 21636579 Free PMC article.
-
The cholinesterase-like domain, essential in thyroglobulin trafficking for thyroid hormone synthesis, is required for protein dimerization.J Biol Chem. 2009 May 8;284(19):12752-61. doi: 10.1074/jbc.M806898200. Epub 2009 Mar 9. J Biol Chem. 2009. PMID: 19276074 Free PMC article.
-
Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer.J Biol Chem. 2010 Jun 4;285(23):17564-73. doi: 10.1074/jbc.M110.111641. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353937 Free PMC article.
-
Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology.Endocr Rev. 2016 Feb;37(1):2-36. doi: 10.1210/er.2015-1090. Epub 2015 Nov 23. Endocr Rev. 2016. PMID: 26595189 Free PMC article. Review.
-
Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones.Endocr Rev. 1998 Apr;19(2):173-202. doi: 10.1210/edrv.19.2.0327. Endocr Rev. 1998. PMID: 9570036 Review.
Cited by
-
Phylogenetic analysis of the human thyroglobulin regions.Thyroid Res. 2012 May 1;5(1):3. doi: 10.1186/1756-6614-5-3. Thyroid Res. 2012. PMID: 22549183 Free PMC article.
-
Targeted Next-Generation Sequencing of Congenital Hypothyroidism-Causative Genes Reveals Unexpected Thyroglobulin Gene Variants in Patients with Iodide Transport Defect.Int J Mol Sci. 2022 Aug 17;23(16):9251. doi: 10.3390/ijms23169251. Int J Mol Sci. 2022. PMID: 36012511 Free PMC article.
-
Repeat motif-containing regions within thyroglobulin.J Biol Chem. 2011 Jul 29;286(30):26327-33. doi: 10.1074/jbc.M111.242099. Epub 2011 Jun 2. J Biol Chem. 2011. PMID: 21636579 Free PMC article.
-
Importance of molecular genetic analysis in the diagnosis and classification of congenital hypothyroidism.Endocrine. 2014 Mar;45(2):163-4. doi: 10.1007/s12020-013-0075-z. Epub 2013 Oct 16. Endocrine. 2014. PMID: 24129411 No abstract available.
-
Thyroglobulin Interactome Profiling Defines Altered Proteostasis Topology Associated With Thyroid Dyshormonogenesis.Mol Cell Proteomics. 2021;20:100008. doi: 10.1074/mcp.RA120.002168. Epub 2020 Dec 8. Mol Cell Proteomics. 2021. PMID: 33581410 Free PMC article.
References
-
- Di Jeso, B., and Arvan, P. 2004. Thyroglobulin structure, function, and biosynthesis. InThe thyroid. L.E. Braverman, and R. Utiger, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 77–95.
-
- den Hartog M.T., Sijmons C.C., Bakker O., Ris-Stalpers C., de Vijlder J.J. Importance of the content and localization of tyrosine residues for thyroxine formation within the N-terminal part of human thyroglobulin. Eur. J. Endocrinol. 1995;132:611–617. - PubMed
-
- Arvan P., et al. Intracellular protein transport to the thyrocyte plasma membrane: potential implications for thyroid physiology. Thyroid. 1997;7:89–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

