Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial
- PMID: 18596969
- PMCID: PMC2432040
- DOI: 10.1371/journal.pone.0002611
Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial
Abstract
Background: The options for medical use of signaling molecules as stimulators of tissue regeneration are currently limited. Preclinical evidence suggests that fibroblast growth factor (FGF)-2 can promote periodontal regeneration. This study aimed to clarify the activity of FGF-2 in stimulating regeneration of periodontal tissue lost by periodontitis and to evaluate the safety of such stimulation.
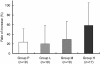
Methodology/principal findings: We used recombinant human FGF-2 with 3% hydroxypropylcellulose (HPC) as vehicle and conducted a randomized double-blinded controlled trial involving 13 facilities. Subjects comprised 74 patients displaying a 2- or 3-walled vertical bone defect as measured > or = 3 mm apical to the bone crest. Patients were randomly assigned to 4 groups: Group P, given HPC with no FGF-2; Group L, given HPC containing 0.03% FGF-2; Group M, given HPC containing 0.1% FGF-2; and Group H, given HPC containing 0.3% FGF-2. Each patient underwent flap operation during which we administered 200 microL of the appropriate investigational drug to the bone defect. Before and for 36 weeks following administration, patients underwent periodontal tissue inspections and standardized radiography of the region under investigation. As a result, a significant difference (p = 0.021) in rate of increase in alveolar bone height was identified between Group P (23.92%) and Group H (58.62%) at 36 weeks. The linear increase in alveolar bone height at 36 weeks in Group P and H was 0.95 mm and 1.85 mm, respectively (p = 0.132). No serious adverse events attributable to the investigational drug were identified.
Conclusions: Although no statistically significant differences were noted for gains in clinical attachment level and alveolar bone gain for FGF-2 groups versus Group P, the significant difference in rate of increase in alveolar bone height (p = 0.021) between Groups P and H at 36 weeks suggests that some efficacy could be expected from FGF-2 in stimulating regeneration of periodontal tissue in patients with periodontitis.
Trial registration: ClinicalTrials.gov NCT00514657.
Conflict of interest statement
Figures



References
-
- Nishihara T, Koseki T. Microbial etiology of periodontitis. Periodontology 2000. 2004;36:14–26. - PubMed
-
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology 2000. 2002;28(1):12–55. - PubMed
-
- Ezzo PJ, Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontology 2000. 2003;32(1):24–35. - PubMed
-
- Kinane DF. Causation and pathogenesis of periodontal disease. Periodontology 2000. 2001;25(1):8–20. - PubMed
-
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

