Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke
- PMID: 18596986
- PMCID: PMC2398740
- DOI: 10.1172/JCI34363
Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke
Abstract
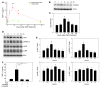
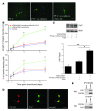
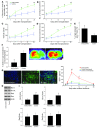
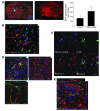
Murine olfactory ensheathing cells (OECs) promote central nervous system axonal regeneration in models of spinal cord injury. We investigated whether OECs could induce a neuroplastic effect to improve the neurological dysfunction caused by hypoxic/ischemic stress. In this study, human OECs/olfactory nerve fibroblasts (hOECs/ONFs) specifically secreted trophic factors including stromal cell-derived factor-1alpha (SDF-1alpha). Rats with intracerebral hOEC/ONF implantation showed more improvement on behavioral measures of neurological deficit following stroke than control rats. [18F]fluoro-2-deoxyglucose PET (FDG-PET) showed increased glucose metabolic activity in the hOEC/ONF-treated group compared with controls. In mice, transplanted hOECs/ONFs and endogenous homing stem cells including intrinsic neural progenitor cells and bone marrow stem cells colocalized with specific neural and vascular markers, indicating stem cell fusion. Both hOECs/ONFs and endogenous homing stem cells enhanced neuroplasticity in the rat and mouse ischemic brain. Upregulation of SDF-1alpha and CXCR4 in hOECs/ONFs promoted neurite outgrowth of cocultured primary cortical neurons under oxygen glucose deprivation conditions and in stroke animals through upregulation of cellular prion protein (PrP C) expression. Therefore, the upregulation of SDF-1alpha and the enhancement of CXCR4 and PrP C interaction induced by hOEC/ONF implantation mediated neuroplastic signals in response to hypoxia and ischemia.
Figures


Similar articles
-
Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases.J Mol Med (Berl). 2009 Dec;87(12):1179-89. doi: 10.1007/s00109-009-0528-2. Epub 2009 Sep 10. J Mol Med (Berl). 2009. PMID: 19756447 Review.
-
Calponin is expressed by fibroblasts and meningeal cells but not olfactory ensheathing cells in the adult peripheral olfactory system.Glia. 2007 Jan 15;55(2):144-51. doi: 10.1002/glia.20443. Glia. 2007. PMID: 17078028
-
Alignment of glial cells stimulates directional neurite growth of CNS neurons in vitro.Neuroscience. 2004;125(3):591-604. doi: 10.1016/j.neuroscience.2004.02.010. Neuroscience. 2004. PMID: 15099673
-
SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model.Brain Res. 2008 Feb 21;1195:104-12. doi: 10.1016/j.brainres.2007.11.068. Epub 2007 Dec 14. Brain Res. 2008. PMID: 18206136
-
The potential therapeutic applications of olfactory ensheathing cells in regenerative medicine.Cell Transplant. 2014;23(4-5):567-71. doi: 10.3727/096368914X678508. Cell Transplant. 2014. PMID: 24816451 Review.
Cited by
-
Progress in stem cell therapy for major human neurological disorders.Stem Cell Rev Rep. 2013 Oct;9(5):685-99. doi: 10.1007/s12015-013-9443-6. Stem Cell Rev Rep. 2013. PMID: 23681704 Review.
-
Novel Programmed Death Ligand 1-AKT-engineered Mesenchymal Stem Cells Promote Neuroplasticity to Target Stroke Therapy.Mol Neurobiol. 2024 Jul;61(7):3819-3835. doi: 10.1007/s12035-023-03779-w. Epub 2023 Nov 29. Mol Neurobiol. 2024. PMID: 38030932
-
Growth factors in ischemic stroke.J Cell Mol Med. 2011 Aug;15(8):1645-87. doi: 10.1111/j.1582-4934.2009.00987.x. Epub 2009 Dec 8. J Cell Mol Med. 2011. PMID: 20015202 Free PMC article. Review.
-
Human Olfactory Ensheathing Cell Transplantation Improves Motor Function in a Mouse Model of Type 3 Spinocerebellar Ataxia.Cell Transplant. 2017 Oct;26(10):1611-1621. doi: 10.1177/0963689717732578. Cell Transplant. 2017. PMID: 29251109 Free PMC article.
-
Brain repair: cell therapy in stroke.Stem Cells Cloning. 2014 Feb 21;7:31-44. doi: 10.2147/SCCAA.S38003. eCollection 2014. Stem Cells Cloning. 2014. PMID: 24627643 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

