Asymmetric insertion of membrane proteins in lipid bilayers by solid-state NMR paramagnetic relaxation enhancement: a cell-penetrating Peptide example
- PMID: 18597439
- PMCID: PMC4083468
- DOI: 10.1021/ja802383t
Asymmetric insertion of membrane proteins in lipid bilayers by solid-state NMR paramagnetic relaxation enhancement: a cell-penetrating Peptide example
Abstract
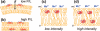
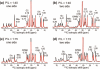
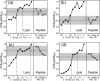
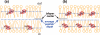
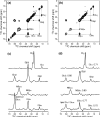
A novel solid-state NMR technique for identifying the asymmetric insertion depths of membrane proteins in lipid bilayers is introduced. By applying Mn (2+) ions on the outer but not the inner leaflet of lipid bilayers, the sidedness of protein residues in the lipid bilayer can be determined through paramagnetic relaxation enhancement (PRE) effects. Protein-free lipid membranes with one-side Mn (2+)-bound surfaces exhibit significant residual (31)P and lipid headgroup (13)C intensities, in contrast to two-side Mn (2+)-bound membranes, where lipid headgroup signals are mostly suppressed. Applying this method to a cell-penetrating peptide, penetratin, we found that at low peptide concentrations, penetratin is distributed in both leaflets of the bilayer, in contrast to the prediction of the electroporation model, which predicts that penetratin binds to only the outer lipid leaflet at low peptide concentrations to cause an electric field that drives subsequent peptide translocation. The invalidation of the electroporation model suggests an alternative mechanism for intracellular import of penetratin, which may involve guanidinium-phosphate complexation between the peptide and the lipids.
Figures

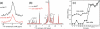
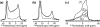
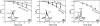
References
-
- von Heijne G. Nat. Rev. Mol. Cell Biol. 2006;7:909–918. - PubMed
-
- Kumashiro KK, Schmidt-Rohr K, Murphy OJ, Ouellette KL, Cramer WA, Thompson LK. J. Am. Chem. Soc. 1998;120:5043–5051.
-
- Huster D, Yao XL, Hong M. J. Am. Chem. Soc. 2002;124:874–883. - PubMed
-
- Luo W, Hong M. Solid State NMR. 2006;29:163–169. - PubMed
-
- Etzkorn M, Martell S, Andronesi OC, Seidel K, Engelhard M, Baldus M. Angew. Chem., Int. Ed. 2007;46:459–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

