Defining stem and progenitor cells within adipose tissue
- PMID: 18597617
- PMCID: PMC2865901
- DOI: 10.1089/scd.2008.0117
Defining stem and progenitor cells within adipose tissue
Abstract
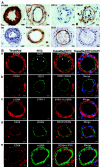
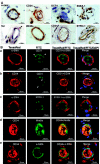
Adipose tissue-derived stem cells (ADSC) are routinely isolated from the stromal vascular fraction (SVF) of homogenized adipose tissue. Freshly isolated ADSC display surface markers that differ from those of cultured ADSC, but both cell preparations are capable of multipotential differentiation. Recent studies have inferred that these progenitors may reside in a perivascular location where they appeared to coexpress CD34 and smooth muscle actin (alpha-SMA) but not CD31. However, these studies provided only limited histological evidence to support such assertions. In the present study, we employed immunohistochemistry and immunofluorescence to define more precisely the location of ADSC within human adipose tissue. Our results show that alpha-SMA and CD31 localized within smooth muscle and endothelial cells, respectively, in all blood vessels examined. CD34 localized to both the intima (endothelium) and adventitia neither of which expressed alpha-SMA. The niche marker Wnt5a was confined exclusively to the vascular wall within mural smooth muscle cells. Surprisingly, the widely accepted mesenchymal stem cell marker STRO-1 was expressed exclusively in the endothelium of capillaries and arterioles but not in the endothelium of arteries. The embryonic stem cell marker SSEA1 localized to a pericytic location in capillaries and in certain smooth muscle cells of arterioles. Cells expressing the embryonic stem cell markers telomerase and OCT4 were rare and observed only in capillaries. Based on these findings and evidence gathered from the existing literature, we propose that ADSC are vascular precursor (stem) cells at various stages of differentiation. In their native tissue, ADSC at early stages of differentiation can differentiate into tissue-specific cells such as adipocytes. Isolated, ADSC can be induced to differentiate into additional cell types such as osteoblasts and chondrocytes.
Figures




References
-
- Helder MN. Knippenberg M. Klein-Nulend J. Wuisman PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799–1808. - PubMed
-
- Schaffler A. Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. - PubMed
-
- Housman TS. Lawrence N. Mellen BG. George MN. Filippo JS. Cerveny KA. DeMarco M. Feldman SR. Fleischer AB. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28:971–978. - PubMed
-
- Strem BM. Hicok KC. Zhu M. Wulur I. Alfonso Z. Schreiber RE. Fraser JK. Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

