Transdermal lovastatin enhances fracture repair in rats
- PMID: 18597639
- PMCID: PMC2685484
- DOI: 10.1359/jbmr.080603
Transdermal lovastatin enhances fracture repair in rats
Abstract
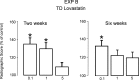
Statins have been shown to stimulate BMP2 transcription and bone formation. This raises the possibility that they could be useful for enhancing rates of fracture repair. Observational studies in patients treated with oral statins for lipid-lowering have been controversial. The likely reason for their inconsistent effects is that the statin concentration reaching the periphery was too low after oral administration to produce a reproducible biologic effect. Thus, we examined the effects of lovastatin (LV) given transdermally in a well-described preclinical model of fracture repair. Effects on the healing fracture callus were assessed by biomechanical strength, radiographs, and quantitative morphology. LV was administered transdermally (TD) for 5 days after fracture in several doses (0.1-5 mg/kg/d) and compared with vehicle-treated control rats and rats treated with LV by oral gavage (PO) at 5-25 mg/kg/d for 5 days from the day of fracture. Radiological evaluation of bones treated with TD LV showed enhanced fracture repair at 2 and 6 wk. BMD in the callus area at 6 wk was also increased in the TD group compared with vehicle-treated controls (p < 0.05). The force required to break TD-treated bones (0.1 mg/kg/d for 5 days) was 42% greater than vehicle-treated controls (p < 0.02), and there was a 90% increase in stiffness (p < 0.01). PO LV at much higher doses (10 and 25 mg/kg/d) showed increased stiffness but no change in other biomechanical properties. By histological examination, a significant increase was also observed in the size of the callus, surrounding proliferating cell nuclear antigen-positive cells, and osteoblast and osteoclast number in TD-treated rats compared with controls at day 8 after fracture (n = 6). In summary, we found that TD LV in low doses accelerates fracture healing, whereas 10-fold the lipid-lowering dose was required to produce any effect when it was administered orally. These studies provide valuable information on the potential of statins and TD delivery as a new and effective therapeutic modality in fracture repair.
Figures




Similar articles
-
Locally delivered lovastatin nanoparticles enhance fracture healing in rats.J Orthop Res. 2007 Oct;25(10):1351-7. doi: 10.1002/jor.20391. J Orthop Res. 2007. PMID: 17506505
-
Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34).J Bone Miner Res. 2002 Nov;17(11):2038-47. doi: 10.1359/jbmr.2002.17.11.2038. J Bone Miner Res. 2002. PMID: 12412812
-
Targeted delivery of lovastatin and tocotrienol to fracture site promotes fracture healing in osteoporosis model: micro-computed tomography and biomechanical evaluation.PLoS One. 2014 Dec 19;9(12):e115595. doi: 10.1371/journal.pone.0115595. eCollection 2014. PLoS One. 2014. PMID: 25526611 Free PMC article.
-
Rats treated with AZD2858, a GSK3 inhibitor, heal fractures rapidly without endochondral bone formation.Bone. 2013 May;54(1):126-32. doi: 10.1016/j.bone.2013.01.019. Epub 2013 Jan 19. Bone. 2013. PMID: 23337038
-
β-Caryophyllene and Statins in Bone Fracture Healing - A Narrative Review.Orthop Res Rev. 2025 Jan 23;17:31-42. doi: 10.2147/ORR.S506427. eCollection 2025. Orthop Res Rev. 2025. PMID: 39872403 Free PMC article. Review.
Cited by
-
Influence of statins locally applied from orthopedic implants on osseous integration.BMC Musculoskelet Disord. 2012 Oct 26;13:208. doi: 10.1186/1471-2474-13-208. BMC Musculoskelet Disord. 2012. PMID: 23102098 Free PMC article.
-
STATINS AND BONE HEALTH: A MINI REVIEW.Actual osteol. 2018 Jan-Apr;14(1):31-35. Actual osteol. 2018. PMID: 30237809 Free PMC article.
-
Saturated fatty acids enhance osteoclast survival.J Lipid Res. 2010 May;51(5):892-9. doi: 10.1194/jlr.M800626. J Lipid Res. 2010. PMID: 20388920 Free PMC article.
-
Germline deletion of AMP-activated protein kinase beta subunits reduces bone mass without altering osteoclast differentiation or function.FASEB J. 2010 Jan;24(1):275-85. doi: 10.1096/fj.09-137158. Epub 2009 Sep 1. FASEB J. 2010. PMID: 19723702 Free PMC article.
-
Lansoprazole Upregulates Polyubiquitination of the TNF Receptor-Associated Factor 6 and Facilitates Runx2-mediated Osteoblastogenesis.EBioMedicine. 2015 Nov 17;2(12):2046-61. doi: 10.1016/j.ebiom.2015.11.024. eCollection 2015 Dec. EBioMedicine. 2015. PMID: 26844285 Free PMC article.
References
-
- Bax BE, Wozney JM, Ashhurst DE. Bone morphogenetic protein-2 increases the rate of callus formation after fracture of the rabbit tibia. Calcif Tissue Int. 1999;65:83–89. - PubMed
-
- Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: A biomechanical and histological study in rats. Bone. 2002;30:816–822. - PubMed
-
- Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85:1425–1435. - PubMed
-
- Hollinger JO, Leong K. Poly(alpha-hydroxy acids): Carriers for bone morphogenetic proteins. Biomaterials. 1996;17:187–194. - PubMed
-
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. - PubMed

